Objectives:
- Explore the data for safety and efficacy of urgent blood pressure lowering in hemorrhagic stroke.
- Is there any benefit from management of elevated blood pressure in acute ischemic stroke that is not a candidate for tPA?
- What is the data for blood pressure target in subarachnoid hemorrhage awaiting definitive management?
PART 1: Hemorrhagic stroke
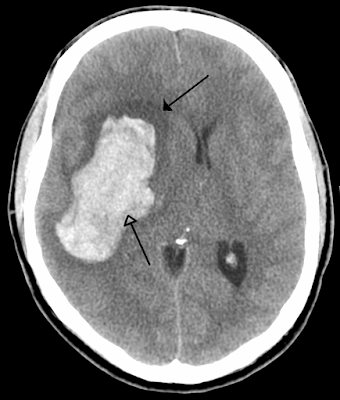
Acute hemorrhagic stroke, which is the least treatable form of stroke, affects more than 1 million people worldwide annually, with the outcome determined by the volume and growth of the underlying hematoma. Blood pressure often becomes elevated after intracerebral hemorrhage (ICH), frequently reaching very high levels, and is associated with poor outcomes.
There have been two major trials looking at modifying BP in ICH, ATACH2 in 2016
and INTERACT2 in 2013.
Background:
- Elevated BP is very common in acute hemorrhagic stroke
- There is an association with elevated BP and:
- Increase in hematoma size
- Neuro-deterioration
- Dependency
- Death
In terms of guidance, the 2015 AHA guidelines are mainly based on the 2013 Interact 2 study. They recommend that for patients presenting with a systolic BP 150-220 that you aim for a systolic reduction to less than 140 and that this might be a reasonable option for patients who present with a BP > 220 as well.
There are 2 main questions which studies have looked at in regards to BP reduction in hemorrhagic stroke and for various neurological conditions in general. They include:
1. Is blood pressure reduction safe?
2. Does BP reduction improve outcomes?
Safety:
In animal models of ICH and human imaging studies there is a zone of hypoperfusion surrounding a clot. So the questions is, will rapid reduction of BP in ICH result in infarction around the area of hemorrhage? There have been several studies which have looked at this question.
SAMURAI Study:
They concluded that high SBP after initiation of standardized antihypertensive treatment was independently associated with neurological deterioration, hematoma expansion, and unfavourable functional outcome in acute ICH. A mean SBP ≈130 mm Hg was associated with the lowest odds ratios for worse clinical outcomes.
There are a lot of limitations of this non-randomized study but they theorized that aggressive antihypertensive treatment for such patients MAY improve clinical outcomes.
ICH ADAPT:
The ICH ADAPT study was a multi-centre, prospective, randomized, open-label, with blinded evaluation study. One of the authors was Dr. Dowlatshahi one of our stroke neurologist here at the The Ottawa Hospital who has a special interest in ICH. We will get to some of his recommendations later on.
In this study eligible patients were those with spontaneous ICH diagnosed on CT < 24 hours after onset with SBP ≥150 mm Hg. Patients with evidence of secondary ICH (eg, vascular malformation), planned surgical resection, or contraindications to CT perfusion were excluded.
Their hypothesis was that early acute BP reduction would not result in significantly lower peri-hematoma CBF compared to patients managed conservatively.
Their primary outcome was to look at CBF in the peri-hematomal region using CT-perfusion scans in an aggressively treated vs non aggressively treated group. They looked at secondary end points of clinical outcomes and ICH expansion but this was a small study and was not powered to detect any differences
This study essentially showed no difference in perihematomal CBF. There was no statistical difference in clinical outcomes or ICH size increase which is maybe not surprising given their small numbers.
This study supported that rapid lowering of BP in ICH is likely safe and we needed more trials to figure out if it might be helpful.
INTERACT Trial
It also appeared to reduce hematoma growth in patients treated within 6 hours after the onset of acute ICH.
They randomized patients to guideline therapy based on the 1999 AHA guidelines of systolic BP < 180 vs intensive therapy of sBP < 140 with the goal to reach target within 1 hour and maintain target for 7 days. The primary outcome they were looking at was the size of the hematoma and perihematomal edema.
They did NOT look at clinical outcomes but we will look at their follow up study later (INTERACT-2).
They did find a statistically significantly difference in the hematoma size after 24 and 72 hours. Once again this was a pilot and feasibility study and with no real clinical outcomes this should be interpreted with caution. But these studies do give us some valuable information on the natural clinical course of hematoma formation and growth.
Efficacy
So that brings us to INTERACT 2 which is the largest RCT evaluating the efficacy of intensive BP reduction.
They enrolled 2839 patients with SBP between 150 and 220 mm Hg within 6 hours of ICH.
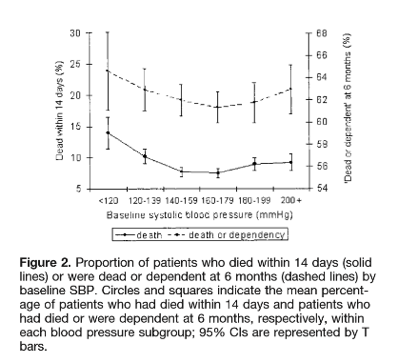
They found that that (52.0%) receiving intensive treatment (to an SBP target of <140 mm Hg within 1 hour of randomization) compared with (55.6%) receiving standard treatment (SBP <180 mm Hg) had a primary outcome of death or major disability (OR, 0.87; 95% CI, 0.75–1.01; P=0.06)
They had a very large sample size with 1399 in the intensive group at 1430 in the guideline based group. The majority of the patients in the study were recruited in China and as you would expect most had a history of HTN. The mean age was 63 years old with a mean systolic BP of 179. Median NIHSS score was 10 and median GCS was 14.
The most common agent used was an alpha blocker which is only available in China called Urapadil, in terms of how sick the groups were in regards to ICU admission and surgical interventions the two groups were very similar.
Looking at the above graph, you can see that at 1 hour, the mean systolic blood pressure was 150 mm Hg in the intensive-treatment group (with 1/3 achieving the target blood pressure of <140 mm Hg) as compared with 164 mm Hg in the standard-treatment group (a difference of 14 mm Hg, P<0.001).
After 6 hours the guideline group levelled off at around 150 and the intensive group around 140 systolic.
About one third of patients achieved the target SBP level within 1 hour (half achieved the target by 6 hours), and most (75%) presented with mild to moderate size (<20 mL) hematomas.
Outcomes:
For the primary outcome there was a slight trend towards benefit for the intensive group with 52% vs 55.6% of the guideline group suffering death or major disability (p 0.06). There was no difference in Death at 90 days or duration of stay in hospital. If you look through the modified Rankin scores the 2 groups look roughly similar.
So overall, this was a negative study with a trend towards benefit from aggressive treatment. In patients with ICH, intensive BP lowering did not reduce risk of death of major disability.
After the trial was started they decided to include the ordinal analysis of the Rankin Scale which showed a marginally significant result in favour of the intensive group with a p value of 0.04. There were no differences noted in adverse events.
So after this large study it seems like a overall rapid reduction of BP to less < 140 is safe. It is possible there is a slight trend towards decreased disability.
Criticisms
The main criticism of the study was the fact the improvement in functional outcome was based on an ordinal analysis which was only considered after the trial had started. Additionally, the vast majority of patients were from China which may limit generalizability.
The most common IV agent used in the study was Urapadil (alpha blocker) which is not used in North America. It also ended up taking awhile to get patients to target and by the time most patients they reached target it was approximately 8 hours post event.
Looking at the natural progression of ICH it looks like under 6 hours 1/3 of hematomas will expand, after 6 hours the vast majority aren’t going to change, so the delay in reduction could have hidden some benefit.
This data is what the 2015 AHA guidelines are based on, which state:
ICH patients presenting with SBP between 150 and 220 mm Hg and without contraindication to acute BP treatment, acute lowering of SBP to 140 mm Hg is safe (Class I; Level of Evidence A) and can be effective for improving functional outcome (Class IIa; Level of Evidence B).
For ICH patients presenting with SBP >220 mm Hg, it may be reasonable to consider aggressive reduction of BP with a continuous intravenous infusion and frequent BP monitoring (Class IIb; Level of Evidence C).
But there has been another large trial looking at this, the ATACH 2 trial:
- Large randomized, multi-centere, open label, international study
- Like INTERACT, the outcome assessors were blinded
- N was 1000 – stopped early for futility of primary outcome
- Compared intensive 110-139 systolic vs 140-179 systolic
- Initially included from 3 hours of onset, increased to 4.5 hours
- Primary outcome was death or major disability
Their inclusion criteria was anyone over the age of 18 with ICH and a GCS of 6 and above with at least one BP of > 180 systolic. They initially included only patients within 3 hours of onset but stretched that to 4.5 hours.
Their exclusion criteria included:
- sBP >240 x 2 measurements
- known neoplasm/AVM/aneurysm
- trauma
- candidate for immediate neurosurgical intervention
- not a ICU candidate.
This study was more standardized in terms of drug usage with IV nicardipine being used as first line and labetalol added if BP target not reached within 30mins.
They screened 8500 patients and recruited 1000 patients. The initial goal was 1280. The mean SBP on presentation was just over 200 systolic. The baseline characteristics were very similar to both groups.
Comparing this population to the INTERACT study they were less sick with 56% having a GCS of 15.
The INTERACT study also included patients with lower BP whereas to be included in this study your arrival BP had to be >180.
The other very important point here is the mean hematoma volume. Dr Dowlatshahi has looked at a few studies in terms of the natural progression of these small volume hematomas and it seems that under 10 cc these hematomas don’t tend to expand, so they may not be the ones to benefit from rapidly decreasing the BP.

The mean interval between symptom onset and randomization was 182.2 minutes in the intensive-treatment group and 184.7 minutes in the standard-treatment group. So this was good because they got patients early in the window under 6 hours where hematoma expansion occurs.
The mean values of hourly minimum systolic blood pressure for the first 24 hours after randomization according to treatment group are shown above in Figure. 1. The mean systolic blood pressure during the first 2 hours was 128.9 in the intensive treatment group 141.1 in the standard-treatment group.
This is really important, Essentially what this trial was looking at was guideline treatment <140 versus very aggressive therapy. Once people saw it was safe to drop to <140 from the INTERACT study they did so.
Results
This was a negative study which was stopped early for futility for the primary outcome which was death or disability which was seen 38.7% of the intensive group vs 37.7% of the standard group for an Relative Risk of 1.02.
If you look at the remainder of the secondary outcome there was no statistical difference in Death, Neurological deterioration in the first 24 hours or serious adverse events (SAE) within the first 72 hours.
There was a statistical difference in SAE at 3 months which was worse in the intensive group which was driven mainly by worse renal function in that group.
Having a look at the modified Rankin scores between the 2 groups they are roughly comparable.
So overall this study was negative but with a caveat in that the ‘less intensive group’ was actually treated quite aggressively to reduce their BP.
Expert Opinion
From speaking with Dr. Dowlatshahi, one of our Stroke Neurologists at The Ottawa Hospital, his opinion is that, unfortunately, we have 2 negative trials looking at aggressive BP reduction so likely the next practice guidelines will say to aim for BP < 180.
There is a population of patients who likely would benefit from rapid acute BP management. What number to aim for will depend on the patient. If there is a small volume hematoma < 10cc in a stable patient then < 180 is reasonable, If there is a large volume hematoma, if it is near ventricles or the patient is worsening then a more aggressive target would be reasonable: He would choose <160 and states some of his more aggressive colleagues may choose <140.
From an ICU perspective, I spoke with ED physician and intensivist at TOH/The Monfort Hospital, Dr Andy Pan. Obviously sBP has limitations and there has been a fair bit of talk in the ICU world about the use of ICP monitors and cerebral oxygenation monitors. That being said we aren’t going to have the benefit of those in the ED and his target would be to target 140-160.
Recommendations:
- Standard therapy should target systolic BP <180
- Consider <160 in large hematomas/worsening clinically/sick enough for ICU
- I would say the current guidelines have this one incorrect. There is insufficient evidence to rapidly reduce to < 140 systolic
PART 2: Ischemic Stroke
What to do about BP control in ischemic stroke that is not TPA eligible?
Blood pressure in ischemic stroke:
- is commonly elevated
- Usually transiently
- Likely secondary to both the stroke and non-stroke factors
Blood pressure is increased in up to 75% to 80% of patients with acute stroke and usually decreases spontaneously over the next few days. This is often due to non-stroke factors such as headache, urinary retention, infection, and psychological stress of admission to hospital as well as disturbed auto-regulation seen with stroke.
Data from observational studies have consistently shown that raised blood pressure after stroke is associated with poor short and long-term outcomes. In patients with acute ischemic stroke, raised blood pressure is theoretically harmful because it increases the risk of cerebral edema and hemorrhagic transformation in the freshly infarcted brain tissue.
However, there are concerns that lowering blood pressure reduces blood flow from collateral vessels to the ischemic penumbra and increases the size of the brain infarction of the stroke.
Regarding this issue the 2015 AHA Guidelines state:
Do not lower the blood pressure during the initial 24 hours of acute ischemic stroke unless the blood pressure is >220/120 mm Hg or there is a concomitant specific medical condition that would benefit.
Theses are essentially identical to the Canadian Hypertension guidelines which state that you can consider reducing the BP if it is 220/120 by 15-25% over the first 24 hours.
Let’s have a look at the evidence. There are some relatively recent studies which may be relevant to the emergency department as they started BP control acutely in the course of ischemic stroke.
- 2011 Lancet Study
- 9 Northern European Countries
- Double blind RCT
- Looked at candesartan vs placebo in acute ischemic and hemorrhagic stroke
SCAST was a large, multicenter, randomized placebo controlled, double-blind trial that enrolled patients with acute ischemic (85%) or hemorrhagic (14%) stroke and systolic blood pressure of above 140 mm Hg.
A total of 2029 patients were randomly assigned within 30 hours of symptom onset (average 18 hours) to either candesartan cilexetil (1017 patients) or placebo (1012 patients) for 7 days with doses increasing from 4 mg on Day 1 to 16 mg on Days 3 to 7.
Overall this study showed no difference in composite end point of stroke, myocardial infarction, or vascular death and analyses of functional outcomes suggested a trend toward higher risk of poor outcome in the candesartan group
- Single Blind RCT
- Used multiple antihypertensives
- 2014 study in JAMA
- Primary outcome was death or major disability at discharge or 14 days
- Target reduce BP by 10-25% in first 24 hours
Patients were randomly assigned to receive antihypertensive treatment (aim to lower BP by 10% to 25% within the first 24 hours after randomization, and achieve BP of 140/90mmHg within 7 days and/or to discontinue all antihypertensive medications (control) during hospitalization.
Death or disability did not differ at all between treatment groups with an odds ratio of 1.00 at 14 days or hospital discharge. There was also no difference in death or the secondary composite outcome of death and major disability at 3-month post-treatment follow-up with an odds ratio of 0.99
Conclusions
My conclusion from these studies and the prior data that we have is that there is no evidence to support BP reduction in acute ischemic stroke that isn’t a tPA candidate unless there is another indication such as acute MI, unstable angina, or another clinical reason to reduce BP. If BP is less than 220 systolic or less than 120 diastolic leave it be.
PART 3: Aneurysmal subarachnoid hemorrhage
What should your blood pressure goals be in acute aneurysmal SAH awaiting definitive care?
What do the guidelines say?
AHA Guidelines 2012:
Between the time of aSAH symptom onset and aneurysm obliteration, blood pressure should be controlled with a titratable agent to balance the risk of stroke, hypertension-related rebleeding, and maintenance of cerebral perfusion pressure (Class I; Level of Evidence B).
The magnitude of blood pressure control to reduce the risk of rebleeding has not been established, but a decrease in systolic blood pressure to <160 mm Hg is reasonable (Class IIa; Level of Evidence C).
Goal is to prevent rebleeding. The risk of rebleeding is maximal in the first 12 hours, Recurrence rates between 4% and 13.6% within the first 24 hours.
Most of the data for the systolic target comes from a Japanese study done by Okhuma et al in 2001. They reviewed 273 cases of SAH which were transferred to their center for definitive treatment. Of the 273 patients, 37 had rebleeding. They then compared the rebleed group to the non-rebleed group to see which patient variables were associated with rebleeding.
The mean value of systolic arterial blood pressure in the rebleed group, which was measured within 20 minutes before rebleeding, was 172 mmHg. The mean value of the maximum value of sBP in the non-rebleed group was 153 mmHg which was statistically significantly.
They also looked at the distribution of the values of systolic blood pressure in the rebleed group and in the non-rebleed group were compared at various cutoff points. For BP above 160 the OR was 3.1 of rebleed.
Their conclusion was that systolic BP over 160 was associated with increased risk of rebleeding.
There are no RCTs looking at this so in the meantime we are forced to use this limited observational study as our guide.
Dr. Rory Connolly is a 4th year Emergency Medicine Resident at the University of Ottawa and The Ottawa Hospital. He has an interest in trauma and sports medicine.
Edited, Formatted and References by Dr. Rob Suttie, PGY2, Emergency Medicine
References
1. Zazulia A, Diringer M, Videen T, Adams R, Yundt K, Aiyagari V et al. Hypoperfusion Without Ischemia Surrounding Acute Intracerebral Hemorrhage. Journal of Cerebral Blood Flow & Metabolism. 2001;:804-810.
2. Butcher K, Baird T, MacGregor L, Desmond P, Tress B, Davis S. Perihematomal Edema in Primary Intracerebral Hemorrhage Is Plasma Derived. Stroke. 2004;35(8):1879-1885.
3. Sakamoto Y, Koga M, Yamagami H, Okuda S, Okada Y, Kimura K et al. Systolic Blood Pressure After Intravenous Antihypertensive Treatment and Clinical Outcomes in Hyperacute Intracerebral Hemorrhage: The Stroke Acute Management With Urgent Risk-Factor Assessment and Improvement-Intracerebral Hemorrhage Study. Stroke. 2013;44(7):1846-1851.
4. Butcher K, Jeerakathil T, Hill M, Demchuk A, Dowlatshahi D, Coutts S et al. The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44(3):620-626.
5. Anderson C, Huang Y, Wang J, Arima H, Neal B, Peng B et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. The Lancet Neurology. 2008;7(5):391-399.
6. Anderson C, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C et al. Rapid Blood-Pressure Lowering in Patients with Acute Intracerebral Hemorrhage. New England Journal of Medicine. 2013;368(25):2355-2365.
7. Qureshi A, Palesch Y, Barsan W, Hanley D, Hsu C, Martin R et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. New England Journal of Medicine. 2016;375(11):1033-1043.
8. Sandset E, Bath P, Boysen G, Jatuzis D, Kõrv J, Lüders S et al. The angiotensin-receptor blocker candesartan for treatment of acute stroke (SCAST): a randomised, placebo-controlled, double-blind trial. The Lancet. 2011;377(9767):741-750.
9. He J, Zhang Y, Xu T, Zhao Q, Wang D, Chen C et al. Effects of Immediate Blood Pressure Reduction on Death and Major Disability in Patients With Acute Ischemic Stroke. JAMA. 2014;311(5):479.