There were some notable papers published in trauma over the past 12 months or so. Here’s a look at three of them, which might help answer a few key questions in trauma management:
- When should you order a CT chest?
- What blood products should we be giving to trauma patients?
- Should we be giving mannitol or hypertonic saline to severe traumatic brain injury?
CLINICAL DECISION RULES FOR OBTAINING A CT CHEST
Derivation and Validation of Two Decision Instruments for Selective Chest CT in Blunt Trauma
- We want to ensure we are not missing significant thoracic injuries in trauma patients
- However, CT chests take time, cost money, and expose patient to significant amounts of radiation.
- A chest CT is about 7 mSv of radiation, (compared to 14 mSv for an abdo/pelvis CT), which is roughly equivalent to 70 PA and lateral CXRs, or 350 trans-Atlantic flights
- In a US study of nearly 10 000 blunt trauma patients, the yield of CT chest for detecting any thoracic injury after a normal CXR was 15%, with a total radiation dose of 59 mSv per injury, and a total radiation dose of 593 mSv per clinically major thoracic injury.
- Researchers have attempted coming up with clinical decision rules about obtaining chest CTs in trauma before, but they have been limited either by the need for multiple other plain x-rays, or by being small, retrospective rules that might be difficult to apply prospectively.
- How can we select which blunt trauma patients require a CT chest, and which are suitable for CXR alone?
What Does This Paper Tell Us?
- Derivation and validation of 2 CDRs at 8 trauma centres (California, NJ, and Boston) from 2011-2014
- Inclusion criteria:
- Over 14 yrs of age
- Presented to ED with blunt trauma <6hrs
- Any chest imaging (CXR or CT chest) ordered in ED at discretion of providers
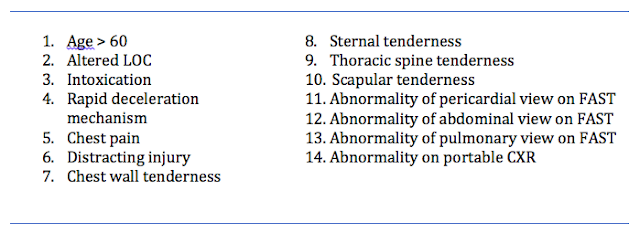
- Study sheets provided MDs with a list of 14 candidate CDR criteria (based on reviews of the literature and investigator consensus):
- Targeted sensitivities of 98% for major injury, and 95% for all injuries (with a 95% CI of +/- 2%)
- Assumed prevalence rates of 5% for major injury and 10% for all injuries
- calculated need to enrol 4570 patients (ended up enrolling 11 477 as a buffer)
Derivation and validation:
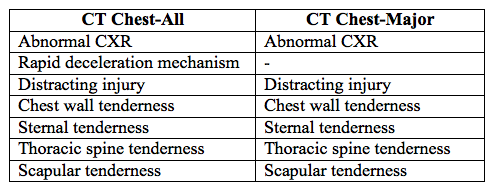
- Chest CT for all injuries (CT Chest-All) was derived first using recursive partitioning, with a sensitivity of 99% for major injuries, and 95% for all injuries
- Then criteria were removed to derive Chest CT-Major, with a sensitivity of 99% for major injury, but a lower acceptable threshold (90%) for all injuries
- Primary analysis was done in the group of patients that received both CXR and CT chest (31.2% in the derivation phase)
- However all patients were followed and a sensitivity analysis performed on the entire group of patients who received any imaging during the validation phase.
- Patients who received no imaging were not followed (previous studies having suggested that the rate of injury in this group is negligible)
- Of patients in the derivation phase, 31.2% had both CXR and CT chest
- Rates of injury in this groups
- Major 7.7%
- Minor/insignificant 27%
- Of patients in the validation phase, 48% had both CXR and CT chest
- Rates of injury in this groups
- Major 4.6%
- Minor/insignificant 22.3%
- CDRs:
- CT Chest-All (for detecting major AND minor injuries)
- Sensitivity 95.4%
- Specificity 25.5%
- NPV 93.9%
- CT Chest-Major (for detecting major injuries)
- Sensitivity 99.2%
- Specificity 31.7%
- NPV 99.9%
- In the validation population of this study, the authors predict that use of Chest CT-All would have avoided 25% of chest CTs, and Chest CT-Major would have avoided 37%
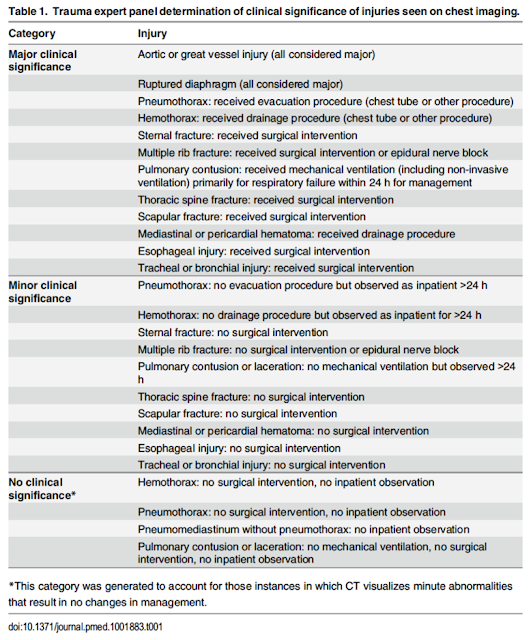
- Injuries missed:
- 1/120 major injuries in this study was not detected by either rule – a PTX that was treated with a chest tube in an 80yo male that fell down the stairs and had a SAH
- 31 minor injuries were missed by Chest CT-All and 64 were missed by Chest CT-Major (mostly rib fractures, sternal fractures, and pulmonary contusions).
- All were non-operative and non-interventional. 9 of these 65 ‘missed’ injuries were sent home directly from the ED, and none returned to the ED
- This is a well-designed prospective derivation and validation of 2 CDRs to help decide what type of imaging a blunt trauma pt should undergo
- The derivation of dual CDRs allows for MDs to practice differently depending on their comfort in missing injuries that do not require intervention
- As per the authors themselves:
- This type of rule should not be used to determine whether a patient needs thoracic imaging or a CT chest in the first place (i.e. as a rule-in, as in some centres using the CDR that way is likely to increase CT use)
- But rather to determine whether you are able to forego a CT chest in a pt whom you have already decided needs some form of thoracic imaging (i.e. as a rule-out).
- In our centre therefore, it may be of less use (as our reflex is likely NOT to perform a CT chest unless there is a strong clinical suspicion)
- Some may disagree with the assessment of major and minor injuries (as some of the minor injuries were still admitted and observed for >24 hrs)
- The rules require external validation prior to wide adoption
Take-home and going forward:
- In blunt trauma patients for whom you have already determined a need for thoracic imaging, but are unsure if a CXR is sufficient, using the Nexus CT-Chest rules may help determine those patients in whom a CXR alone is adequate (though this is going to be a pretty small group, according to the rules).
- However, it is NOT specific enough to help you then determine which patients overall need a CT.
- Development of a ‘rule-in’ CDR that helps determine the more specific factors suggesting a pt needs a CT chest after initial CXR would be helpful
RATIOS OF BLOOD PRODUCTS IN MASSIVE TRANSFUSION
The PROPPR Trial.
- 20-40% of trauma deaths that occur after hospitalization are due to massive haemorrhage
- Improved resuscitation and transfusion techniques may help reduce this rate of mortality
- Traditional resuscitation techniques in haemorrhage involve the administration of crystalloid fluid and PRBCs, often slowly and targeting hemoglobin levels
- ‘Damage control resuscitation’ is the rapid transfusion of blood products (plasma, platelets, and PRBCs) in a balanced ratio (usually 1:1:1) designed to approximate whole blood, as well as the prevention and correction of coagulopathy
- The goal is to better treat intravascular volume deficits, the acute coagulopathy of trauma, to preserve oxygen-carrying capacity, repair the endothelium, and prevent dilutional coagulopathy
- Retrospective military data of 246 trauma patients undergoing massive transfusion has previously shown that a transfusion ratio of 1:8 (plasma to PRBCs) is associated with a 65% mortality rate, compared to a mortality of 19% with ratios of 1:1.4
- Prospective observational data from the civilian world has shown similar, though perhaps less significant trends, with a 30-day mortality of 64% in patients receiving <1:2 (plasma to PRBCs) and 41% in patients receiving >1:2.
- It showed reduced hazard ratios for death within the first 6 hrs when comparing low (<1:2) and high (>1:1) ratios of plasma:PRBCs or platelets:PRBCs (with hazard ratios going as low as 0.23 in the high ratio groups)
- However, the study was limited by poor standardization, and seemingly high heterogeneity in terms of how and when patients got blood products
Does a balanced transfusion strategy affect mortality when studied in a prospective, randomized fashion?
- Randomized trial of 680 trauma patients requiring trauma team activation, administration of at least one unit of blood in first hour, and predicted to require massive transfusion (either by MD judgement or using the Assessment of Blood Consumption score) at 12 different Level 1 trauma centres in North America
- Excluded pregnancy, burns, inhalational injuries, CPR, and (most significantly) those who were not expected to survive past 1 hr on arrival to the ED.
- The two arms were composed of a 1:1:1 ratio and 1:1:2 ratio based on the PROMMTT study (which showed that clinicians clustered around these ratios anyways)
- All containers included: 6 units of plasma, 1 dose of platelets (6 units on average), and 6 units of PRBCs
- Transfused in the following order: platelets first, then alternating RBC and plasma
1:1:2 protocol (composition and order changed from odd to even containers):
- Odd-numbered containers included: 3 units of plasma, 0 doses of platelets, 6 units of PRBCs
- Transfused in the following order: 2 units of PRBCs then 1 unit of plasma (repeated x 2)
- Even-numbered containers included: 3 units of plasma, 1 dose of platelets (6 units on average), and 6 units of PRBCs
- Transfused in the following order: platelets first, then alternating 2 units of PRBCs and 1 unit of plasma
- Stopping transfusion was as clinically indicated
- 24hr and 30 day mortality
- Time to hemostasis (either judged by surgeon or in IR suite)
- Number and type of blood products used before hemostasis was achieved, and after hemostasis up the first 24hrs
- Complications
- Hospital, ventilator and ICU-free days
- Incidence of surgery
- Functional status at discharge/30 days
- 4 patients lost to 30 day follow-up: sensitivity analysis for all possible outcomes was computed and a range of values incorporated
- P values were adjusted for 2 interim analyses (p=0.044)
- 11 185 patients assessed for eligibility, 10 505 excluded (most did not receive at least one unit of blood, were not direct transfers, or were not predicted to need massive transfusion – 130 were excluded because they were expected to die within an hr)
- 75% of patients required IR or OR within 2hrs
- 24hr mortality:
- 1:1:1 – 12.7%
- 1:1:2 – 17.0% (NS)
- Exsanguination (the main cause of death) in the 1st 24hrs WAS reduced significantly (9.2% vs. 14.6%, p=0.03)
- 30 day mortality:
- 1:1:1 – 22.4%
- 1:1:2 – 26.1% (NS)
- In the 1st 24hrs, the 1:1:1 group received more total blood products (a median of 25.5 units of blood products, compared to 19 units in the other group), but similar total amount of PRBCs
- 1:1:1 got 7 units plasma, 12 units platelets, 9 units PRBCs
- 1:1:2 got 5 units plasma, 6 units platelets, and 9 units PRBCs
- The 1:1:1 group actually ended up receiving something more closely approximating 1:1.5:1 (ie. more platelets)
- The 1:1:2 group actually ended up receiving something more closely approximating 2:1:4 (ie. less platelets)
Take-homes and going forward:
- How can we better predict what patients should be included in a massive transfusion protocol to begin with?
- What is the role of laboratory guided transfusion protocols?
HYPERTONIC SALINE IN SEVERE TRAUMATIC BRAIN INJURY
HTS in Severe TBI: Systematic Review and Meta-analysis
- Increased ICP in severe traumatic brain injury (sTBI) is strongly associated with mortality
- Osmotic agents are used in sTBI with signs of increased ICP such as:
- A herniation syndrome
- Cushing’s reflex
- Progressive neurologic deterioration not attributable to an extracranial cause
- However, it may also have detrimental effects:
- May produce AKI
- May actually produce HYPOtension in large doses (due to diuresis)
- May cause paradoxical bleeding into a cerebral hematoma by reducing the local tamponade
- It may increase systemic MAP, which leads to cerebral vasoconstriction (in a brain where auto-regulation persists), and thus a reduction in ICP
- May also produce AKI
- Risk of cerebral pontine myelinolysis
- Hypernatremia
- May lead to RBC lysis
- May worsen coagulopathy
- The Brain Trauma Foundation and American Association of Neurological Surgeons clinical practice guidelines give a Level II recommendation that mannitol is effective for control of raised ICP.
- They note that current evidence is not sufficient to make recommendations about HTS, as does the 2008 ATLS guidelines
What is the cumulative evidence for using HTS in sTBI
- The largest systematic review and meta-analysis of of RCTs studying HTS in sTBI to date
- Reviewed a wide variety of databases and grey literature, coming up with 11 studies (1820 pts) of adults with a sTBI (GCS <8), randomly assigned to receive HTS vs any other type of solution
- Primary outcomes looked at by the investigators were death and ICP control
- They wanted to look at secondary outcomes of neurological outcome at discharge, LOS, but almost none of the studies reported these
- They also looked at adverse events as reported in the studies
- Used the Cochrane Collaboration tool for risk of bias
- Of the 11 studies, only 3 enrolled more than 100 pts
- Only 2 studies were considered at low risk of overall bias (only missing one of the criteria on the Cochrane risk of bias tool
- All the studies monitored osmolality and natremia, but not in a standardized way that would permit meta-analysis.
- All studies noted some incidence of hypernatremia, but most did not monitor for any related clinical adverse events
Take-homes and going forward:
- What is the ideal concentration of HTS to use? What is the ideal agent in the ED? What effect do either mannitol or HTS actually have on mortality and disability?
References: