With legalization, and increased ease of access to marijuana, there is increased focus on the diagnosis of Cannabinoid Hyperemesis Syndrome, with broader media attention and thus patient awareness to this entity.
History:
- Cannabis (Cannabis sativa spp.) has been harvested for food (seeds), fibre (stems), and medicine (buds) throughout most of human history.
- ~2900 BCE: Chinese Emperor Fu His characterized cannabis as having sacred yin and yang features, suggesting that it could restore homeostasis to an unbalanced body.
- Documented evidence reveals physicians in ancient Egypt, India, Persia, Roma, Arabia, and Greece used cannabis for spiritual and medicinal purposes. Indications included menstrual fatigue, gout, rheumatism, malaria, constipation, pain, and absent-mindedness.
- 1923: cannabis is added to the Opium and Narcotic Drug Act, making it illegal.
- 1960s: with the rise of the hippie movement, cannabis use becomes more popular.
- 2001: Canada becomes the 2nd country to have a government-run medical cannabis program.
- 2018: the Cannabis Act legalizes possession and consumption cannabis in Canada.
Active components:
- Cannabis plants synthesize many cannabinoids in their budding structures, the most abundant of which are-tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol (product of THC degradation).
- The primary psychoactive agent in cannabis is THC.
- In the 1970s to 1990s, circulating cannabis had ~3 – 4% (or 30 – 40mg/g) THC.
- Today, dried flower in Ontario cannabis stores often has up to 30% (300mg/g) THC.
- Concentrated products such as waxes, resin, shatter, and vaping oils have much higher THC concentrations closer to 90% (900mg/g).
- Note that these concentrations refer to the amount of THC per weight in a product at the time of purchase. Often a second concentration value will be listed as the “total THC“, referring to the total amount of active THC when a combusted product is consumed. This is because cannabinoids must be heated to 150°C to be activated, which happens either during processing or when a consumer heats the product.
Absorption:
- Varies depending on route of administration.
- Inhaled cannabis: higher peak serum concentration, faster (3 – 10 min), higher bioavailability (up to 35%).
- PO: reaches peak serum concentrations in ~120 min with lower bioavailability (6%).
- The extensive first pass hepatic metabolism is responsible for relatively low PO bioavailability (Lucas, 2018).
Metabolism:
- THC is metabolized by CYP450 in two main metabolites: 11-hydroxy-THC (hydroxy THC) & THC-COOH (carboxy THC).
- Hydroxy THC is formed quickly, peaking shortly after THC, and exhibits similar psychoactive properties.
- Carboxy THC is not psychoactive and peaks ~2 h after smoking. It is then excreted in urine, which is used for forensic analysis of body fluids (Lucas, 2018).
Distribution:
- THC is highly lipophilic. It can cross into breastmilk and through the placenta. There is very limited and weak evidence on the extent of THC crossing the placenta or any potential detrimental effects to a developing fetus.
- It is recommended that one should abstain from cannabis use while pregnant or chest-feeding (Thompson, 2019).
Cannabinoid Hyperemesis Syndrome (CHS)
First described in 2004 by Allen et al., CHS emerged as a pattern of cyclic vomiting and repetitive hot bathing associated with patients who use cannabis long-term, often with higher frequency. Prevalence is unclear, with 3 – 33 % of those using chronically who suffer from CHS.
Rome 4 diagnostic criteria for CHS:
- Stereotypical episodic vomiting resembling cyclic vomiting syndrome (CVS) in terms of onset, duration, and frequency
- Presentation after prolonged use of cannabis
- Relief of vomiting episodes by sustained cessation of cannabis use
*All criteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis. May be associated with repetitive bathing behaviour (prolonged hot baths or showers).
Revised Criteria by Venkatesan et al. (2019):
In 2019, Venkatesan et al. outlined the limitations of the Rome 4 framework and proposed the following revised criteria:
| Clinical features | Stereotypical episodic vomiting resembling CVS in terms of onset, with ≥3 episodes per year. |
| Cannabis use patterns | Duration of use >1 year preceding onset of symptoms. Frequency of use >4 times per week on average. |
| Cannabis cessation | Resolution of symptoms should follow a period of cessation from cannabis for a minimum of 6 months or at least equal to a duration that spans three typical cycles in an individual patient. |
Other supportive clinical features:
- Usually associated with inhaled cannabis use
- Abdominal pain, often epigastric or periumbilical
- Age < 50 yrs
- Weight loss of >5 kg
- Morning predominance of symptoms
- Normal bowel habits (but keep in mind that diarrhea can ensure from GI irritation)
- Negative laboratory, radiographic, and endoscopic test results
The 3 phases of CHS:
- Prodromal phase: mild nausea, abdominal discomfort, and fear of vomiting with continued use of cannabis and mostly normal eating habits. Typically, weeks to months in length.
- Hyperemetic phase: multiple intense episodes of vomiting, compulsive hot bathing, and severe abdominal pain. This is the phase we are most familiar with in the ED. Duration is controversial, typically lasting 24 – 48 h with active medical management, or until prolonged abstinence from use.
- Recovery phase: restoration of normal eating behaviours and generally feeling well.
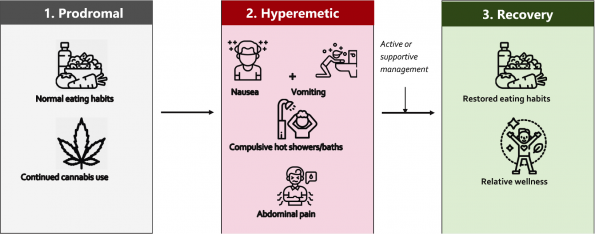
Figure 1 | the three phases of CHS (Zhu et al., 2021).
Pathophysiology:
Three main theories are implicated in CHS:
- Genetic polymorphisms:
- Hepatic cannabinoid metabolizing enzymes in some individuals results in toxic accumulation of metabolites.
- Russo et al. (2022) demonstrated statistically significant mutations in genes coding for COMT, CYP2C9, TRPV1, DRD2, ABCA1 all with the potential to trigger symptoms of CHS.
- Reintoxication:
- Highly-lipophilic THC sits in our adipose tissue.
- Gunasekaran et al. (2009) demonstrated that when rat models are stressed the THC in these stores is released back into the system. This proposed excessive release causes the enteric stimulation of CB1, precipitating symptoms of CHS.
- CB1 action in the GI system:
- THC stimulates the CB1 receptors in the GI tract. This can cause reduced gastric emptying, increased secretions, and lower esophageal relaxation which can lead to nausea and vomiting.
- Cannabis is currently being studied for these effects in GI disorders such as IBD.
These theories are still evolving as we continue to discover more about this disorder. Clinical experience would support the idea that THC stored in adipose tissue can lead to CHS, even when patients have abstained from using marijuana for days at at time.
CHS Workup
Patients with CHS can often develop significant dehydration, and subsequent electrolyte abnormalities and renal injury. It is worth having a low threshold to do basic bloodwork, as well as an ECG in these patients. Especially consider this in patients that have been unwell for a few days, an inability to tolerate oral intake, or previous complications from CHS. The ECG provides an additional layer of comfort when providing treatment (typically Haloperidol) to these patients.
CHS Treatment:
High level evidence for CHS treatment is severely lacking. Very few randomized controlled trials comparing varying treatment arms exist. The majority of evidence is from systematic reviews of case series with varying treatment attempts:
Recommended treatments:
- Haloperidol 0.05 mg/kg IV – FIRST LINE (Ruberto et al., 2021).
- routine use of other antiemetics like ondansetron, metoclopramide, and diphenhydrinate is not recommended, and should be reserved only for patients refractory to other therapies.
- The most common dose used in the literature is 5mg, and is extrapolated from diabetic gastroparesis literature. It appears that the incidence of EPS symptoms is rare at this dose.
- Locally, it appears most providers start with a dose of 2-5 mg IV, and may provide a second dose based on effect. It is not recommended to provide more than 10 mg.
- Capsaicin cream on the abdomen, any strength can be useful, studies most often used 0.1% but there was also a positive effect with concentrations as a low as 0.025% (Dean et al., 2020).
- Lorazepam – weak recommendation for one time ED dose in the pt with severe anxiety surrounding PO intake (Cox et al., 2012).
- Routine use of other benzodiazepines is not recommended.
- Cessation of cannabis use is the only truly proven treatment to resolve CHS.
- Avoid the use of opioids for abdominal pain in this setting.
Reducing harm from cannabis use
Cannabis use disorder (CUD) is an under-recognized risk of cannabis use. Defined by the DSM V criteria of cannabis use disorder, in the emergency department we should be screening for the disorder with questionnaires such as the severity of dependance scale (Martin, 2006) with a score of 3 or greater out of 15 is a positive screen or the CAGE questionnaire adapted for cannabis use where a single yes to any question is a positive screen. Another alternative screening questionnaire is the CUDIT-R. It is also worth the consideration of referral to addiction medicine specialists for the treatment of cannabis use disorder.
Cessation recommendation for cannabis use are simlar to other substance use disorders where behavioral therapy has been proven to be modestly helpful.
Ideally, when marijuana is used inappropriately, cessation is the ideal approach to therapy. However, when patients are not yet pre-contemplative, a harm reduction approach is important to coach patients on:
- CBD > THC
- Use it as a tool, i.e.: do not use marijuana because it is part of a routine, or because one is ‘bored’. Attempt to utilize it to be introspective, be productive, take walks etc.
- Avoid using daily
- Ensure appropriate hydration and caloric intake
- Strongly discourage marijuana usage at a young age
Pregnant women who are using cannabis for symptomatic control (nausea and vomiting) should be counselled on the potential harm to the developing fetus and offered alternatives for hyperemesis gravidarum treatment (Vanstone, 2021).
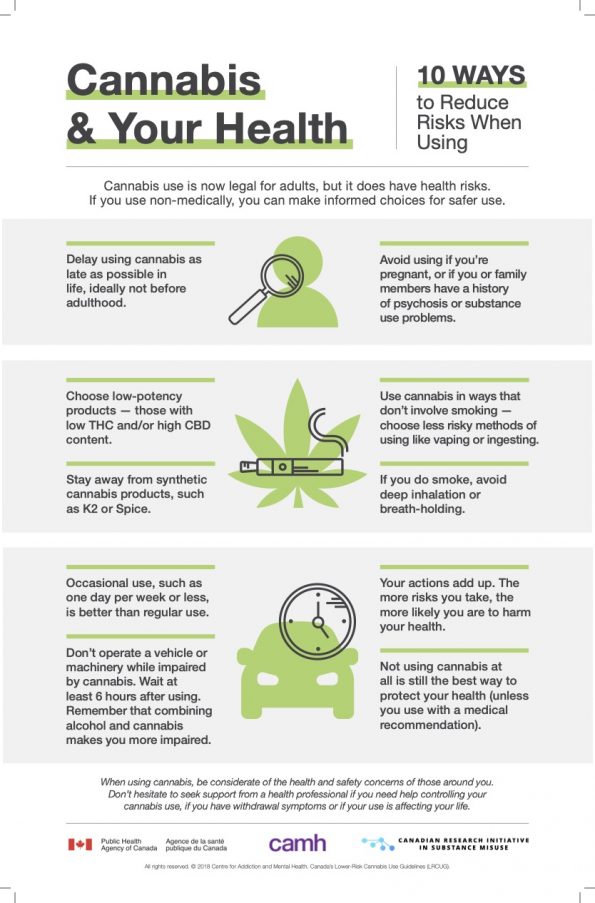
Figure 2 | Cannabis & Your Health – 10 ways to reduce risks when using. Patient handout (PHAC, CAMH, CRISM).
References
- Allen, J. H., De Moore, G. M., Heddle, R., & Twartz, J. C. (2004). Cannabinoid hyperemesis: Cyclical hyperemesis in association with chronic cannabis abuse. Gut, 53(11), 1566–1570. https://doi.org/10.1136/gut.2003.036350
- Ashton, C. H. (2001). Pharmacology and effects of cannabis: A brief review. British Journal of Psychiatry, 178(FEB.), 101–106. https://doi.org/10.1192/bjp.178.2.101
- Blumentrath, C. G., Dohrmann, B., & Ewald, N. (2017). Cannabinoid hyperemesis and the cyclic vomiting syndrome in adults: recognition, diagnosis, acute and long-term treatment. German Medical Science : GMS e-Journal, 15, Doc06. https://doi.org/10.3205/000247
- Burillo-Putze, G., Richards, J. R., Rodríguez-Jiménez, C., & Sanchez-Agüera, A. (2022). Pharmacological management of cannabinoid hyperemesis syndrome: an update of the clinical literature. Expert Opinion on Pharmacotherapy, 23(6), 693–702. https://doi.org/10.1080/14656566.2022.2049237
- Centre, N. C. P. and I. (2006). Severity of Dependence Scale (sds). National Cannabis Prevention and Information Centre, 1998, 2006.
- Chocron, Y., Zuber, J. P., & Vaucher, J. (2019). Cannabinoid hyperemesis syndrome. The BMJ, 366(July), 10–13. https://doi.org/10.1136/bmj.l4336
- Choung, R. S., Locke, G. R., Lee, R. M., Schleck, C. D., Zinsmeister, A. R., & Talley, N. J. (2012). Cyclic vomiting syndrome and functional vomiting in adults: Association with cannabinoid use in males. Neurogastroenterology and Motility, 24(1), 20–27. https://doi.org/10.1111/j.1365-2982.2011.01791.x
- Cox, B., Chhabra, A., Adler, M., Simmons, J., & Randlett, D. (2012). Cannabinoid hyperemesis syndrome: Case report of a paradoxical reaction with heavy marijuana use. Case Reports in Medicine, 2012, 10–12. https://doi.org/10.1155/2012/757696
- Dean, D. J., Sabagha, N., Rose, K., Weiss, A., France, J., Asmar, T., Rammal, J. A., Beyer, M., Bussa, R., Ross, J., Chaudhry, K., Smoot, T., Wilson, K., & Miller, J. (2020). A Pilot Trial of Topical Capsaicin Cream for Treatment of Cannabinoid Hyperemesis Syndrome. Academic Emergency Medicine, 27(11), 1166–1172. https://doi.org/10.1111/acem.14062
- Degenhardt, L., Ferrari, A. J., Calabria, B., Hall, W. D., Norman, R. E., McGrath, J., Flaxman, A. D., Engell, R. E., Freedman, G. D., Whiteford, H. A., & Vos, T. (2013). The Global Epidemiology and Contribution of Cannabis Use and Dependence to the Global Burden of Disease: Results from the GBD 2010 Study. PLoS ONE, 8(10), 9–37. https://doi.org/10.1371/journal.pone.0076635
- Eichhorn Bilodeau, S., Wu, B. S., Rufyikiri, A. S., MacPherson, S., & Lefsrud, M. (2019). An update on plant photobiology and implications for cannabis production. Frontiers in plant science, 10, 296. https://doi.org/10.3389/fpls.2019.00296
- Gunasekaran, N., Long, L. E., Dawson, B. L., Hansen, G. H., Richardson, D. P., Li, K. M., Arnold, J. C., & McGregor, I. S. (2009). Reintoxication: The release of fat-stored Δ 9- tetrahydrocannabinol (THC) into blood is enhanced by food deprivation or ACTH exposure. British Journal of Pharmacology, 158(5), 1330–1337. https://doi.org/10.1111/j.1476-5381.2009.00399.x
- Hernandez, J. M., Paty, J., & Price, I. M. (2018). Cannabinoid hyperemesis syndrome presentation to the emergency department: A two-year multicentre retrospective chart review in a major urban area. Canadian Journal of Emergency Medicine, 20(4), 550–555. https://doi.org/10.1017/cem.2017.381
- Hickey, J. L., Witsil, J. C., & Mycyk, M. B. (2013). Haloperidol for treatment of cannabinoid hyperemesis syndrome. American Journal of Emergency Medicine, 31(6), 1003.e5-1003.e6. https://doi.org/10.1016/j.ajem.2013.02.021
- Hryhorowicz, S., Kaczmarek-Ryś, M., Zielińska, A., Scott, R. J., Słomski, R., & Pławski, A. (2021). Endocannabinoid System as a Promising Therapeutic Target in Inflammatory Bowel Disease – A Systematic Review. Frontiers in Immunology, 12(December), 1–16. https://doi.org/10.3389/fimmu.2021.790803
- I.J., M. (2005). Pharmacokinetics of cannabinoids. Pain Research and Management, 10(SUPPL. A), 15A-22A. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L44796349
- King, C., & Holmes, A. (2015). Cannabinoid hyperemesis syndrome. Cmaj, 187(5), 355. https://doi.org/10.1503/cmaj.140154
- Lam, P. W., & Frost, D. W. (2014). Nabilone therapy for cannabis withdrawal presenting as protracted nausea and vomiting. BMJ Case Reports, 2014, 1–3. https://doi.org/10.1136/bcr-2014-205287
- Lapoint, J., Meyer, S., Yu, C. K., Koenig, K. L., Lev, R., Thihalolipavan, S., Staats, K., & Kahn, C. A. (2018). Cannabinoid hyperemesis syndrome: Public health implications and a novel model treatment guideline. Western Journal of Emergency Medicine, 19(2), 380–386. https://doi.org/10.5811/westjem.2017.11.36368
- Lucas, C. J., Galettis, P., & Schneider, J. (2018). The pharmacokinetics and the pharmacodynamics of cannabinoids. British Journal of Clinical Pharmacology, 84(11), 2477–2482. https://doi.org/10.1111/bcp.13710
- McGolrick, D., & Frey, N. (2018). Nabilone for Chronic Pain Management: A Review of Clinical Effectiveness and Guidelines – An Update. Nabilone for Chronic Pain Management: A Review of Clinical Effectiveness and Guidelines – An Update, 1–24.
- Mehmedic, Z., Chandra, S., Slade, D., Denham, H., Foster, S., Patel, A. S., Ross, S. A., Khan, I. A., & ElSohly, M. A. (2010). Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. Journal of Forensic Sciences, 55(5), 1209–1217. https://doi.org/10.1111/j.1556-4029.2010.01441.x
- Morris, R., & Fisher, M. (2014). Cannabinoid hyperemesis syndrome: A specific cause of cyclical vomiting. International Journal of Adolescent Medicine and Health, 26(1), 153–156. https://doi.org/10.1515/ijamh-2012-0113
- Parker, L. A., Rock, E. M., & Limebeer, C. L. (2011). Regulation of nausea and vomiting by cannabinoids. British Journal of Pharmacology, 163(7), 1411–1422. https://doi.org/10.1111/j.1476-5381.2010.01176.x
- Pergolizzi Jr., J. V., LeQuang, J. A., & Bisney, J. F. (2018). Cannabinoid Hyperemesis. Medical Cannabis and Cannabinoids, 1(2), 73–95. https://doi.org/10.1159/000494992
- Perisetti, A., Gajendran, M., Dasari, C. S., Bansal, P., Aziz, M., Inamdar, S., Tharian, B., & Goyal, H. (2020). Cannabis hyperemesis syndrome: An update on the pathophysiology and management. Annals of Gastroenterology, 33(6), 571–578. https://doi.org/10.20524/aog.2020.0528
- Report, C., Care, P., Good, H., Can, I., & Wrong, G. (2021). Clinics in Oncology A Case Report on Cannabinoid Hyperemesis Syndrome in. 6, 6–9.
- Richards, J. R., Gordon, B. K., Danielson, A. R., & Moulin, A. K. (2017). Pharmacologic Treatment of Cannabinoid Hyperemesis Syndrome: A Systematic Review. Pharmacotherapy, 37(6), 725–734. https://doi.org/10.1002/phar.1931
- Rosenberg, E. C., Tsien, R. W., Whalley, B. J., & Devinsky, O. (2015). Cannabinoids and Epilepsy. Neurotherapeutics, 12(4), 747–768. https://doi.org/10.1007/s13311-015-0375-5
- Ruberto, A. J., Sivilotti, M. L. A., Forrester, S., Hall, A. K., Crawford, F. M., & Day, A. G. (2021). Intravenous Haloperidol Versus Ondansetron for Cannabis Hyperemesis Syndrome (HaVOC): A Randomized, Controlled Trial. Annals of Emergency Medicine, 77(6), 613–619. https://doi.org/10.1016/j.annemergmed.2020.08.021
- Russo, E. B. (2016). The solution to the medicinal cannabis problem. In Ethical Issues in Chronic Pain Management (Issue October). https://doi.org/10.3109/9781420009101-11
- Russo, E. B., Spooner, C., May, L., Leslie, R., & Whiteley, V. L. (2022). Cannabinoid Hyperemesis Syndrome Survey and Genomic Investigation. Cannabis and Cannabinoid Research, 7(3), 336–344. https://doi.org/10.1089/can.2021.0046
- Senderovich, H., Patel, P., Jimenez Lopez, B., & Waicus, S. (2022). A Systematic Review on Cannabis Hyperemesis Syndrome and Its Management Options. Medical Principles and Practice, 31(1), 29–38. https://doi.org/10.1159/000520417
- Simonetto, D. A., Oxentenko, A. S., Herman, M. L., & Szostek, J. H. (2012). Cannabinoid hyperemesis: A case series of 98 patients. Mayo Clinic Proceedings, 87(2), 114–119. https://doi.org/10.1016/j.mayocp.2011.10.005
- Sontineni, S. P., Chaudhary, S., Sontineni, V., & Lanspa, S. J. (2009). Cannabinoid hyperemesis syndrome: Clinical diagnosis of an underrecognised manifestation of chronic cannabis abuse. World Journal of Gastroenterology, 15(10), 1264–1266. https://doi.org/10.3748/wjg.15.1264
- Sorensen, C. J., DeSanto, K., Borgelt, L., Phillips, K. T., & Monte, A. A. (2017). Cannabinoid Hyperemesis Syndrome: Diagnosis, Pathophysiology, and Treatment—a Systematic Review. Journal of Medical Toxicology, 13(1), 71–87. https://doi.org/10.1007/s13181-016-0595-z


