Fluids and antibiotics and lactate…. oh my! Sepsis management in the Emergency Department (ED) was revolutionized by the Rivers trial – a relatively small study showing a NNT of 6 with early goal directed therapy (EGDT). It heavily influenced the end-goals of resuscitation in the Surviving Sepsis Campaign (SSC) that many ED sepsis protocols around the world have adopted. But, what evidence are these recommendations based on?
Here’s an evidence-based bottom line for four end-points of resuscitation in sepsis: lactate, MAP, CVP and IVC ultrasound.
Lactate
The controversy:
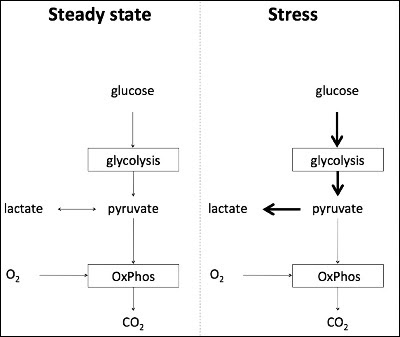
- Under steady state, glycolysis is converted to pyruvate which is used in the Citric Acid Cycle (CAC) and the subsequent electron carriers produced are used in the electron transport chain (ETC) to generate ATP.
- During increased physiological stress, the rate of glycolysis ramps up 10 to 100 times. The ETC and the CAC is unable to use the amount of pyruvate generated fast enough at the rate that its being produced.
- Hence, the pyruvate is metabolized into lactate.
- It has been proposed that lactate is generated via adrenergic stimulation to fuel tissues under physiological stress and not necessarily secondary to lack of tissue oxygenation.
- So, what’s the evidence to support that lactate is associated with tissue hypoxia? Studies have concluded that in the setting of tissue hypoxia there is hyperlactemia, hence we’ve inferred that lactate is a direct indication of tissue hypoxia, but this may be erroneous.
Bottom line:
- Hyperlactemia is strongly associated with increased morbidity and mortality.
- There are alternate mechanisms of lactate production that do not involve oxygen debt.
- Beware of over-resuscitation when aiming for normalization of lactate and using it in isolation as an end-point of resuscitation.
- SCC guidelines suggest targeting normalization of lactate.
Mean Arterial Pressure (MAP)
Background: The MAP is the average pressure driving systemic perfusion. Normally organs are able to autoregulate their perfusion over a wide range of blood pressure. However, this ability to autoregulate is lost in patients with septic shock. Based on this, it has previously been suggested that an increase in blood pressure will result in increased organ perfusion.
The controversy:
- The “magic number” of a MAP of 65 comes from multiple studies, (some clinical and other animal studies) that demonstrated a MAP < 60 mm Hg is generally associated with worse outcomes.
- The Rivers trial based its MAP recommendation on two small retrospective studies that showed no improvement in lactate clearance with targeting a higher MAP.
- The SEPSISSPAM study is the biggest RCT to date that targeted a high (75-85 mm Hg) versus low (65-75 mm Hg) MAP in patients with septic shock – and found that there was no difference in all-cause mortality at 28 days.
- However, this RCT did show that targeting a higher MAP in those with a history of HTN decreases the incidence of AKI. We already know from existing evidence that renal replacement therapy is associated with higher mortality.
Bottom Line:
- No difference in high versus low MAP targets in patients with septic shock.
- Knowing your patient’s baseline BP may be helpful in knowing what MAP to target – ie: low baseline BP, then targeting even a MAP of 65 mm Hg may be too high, conversely, for a patient with hypertension, consider targeting a higher MAP.
- Individualized therapy should be considered instead of a protocol-based approach.
- SSC Guidelines suggest targeting a MAP of ≥ 65 mm Hg.
Central Venous Pressure (CVP)
CVP measurement for fluid status is based on the dogma that low CVP equates to a patient who is volume depleted, and high CVP suggests volume overload. But does CVP actually correlate with fluid status? Two systematic reviews by Marik et al., concluded that there was no association between CVP and circulating blood volume.
SCC Guidelines suggest using CVP (in addition to ScvO2 and beside ECHO) in assessing volume status and tissue perfusion.
Bottom line:
- Variability in CVP measurement.
- Lack of evidence to support its use, particularly in the acute setting.
Inferior Vena Cava (IVC) Ultrasound
After CVP slowly fell out of favour, IVC ultrasound was introduced as the ultimate, easy and non-invasive method of assessing fluid status in the ED in patients presenting with shock. There is evidence for the use of IVC ultrasound in mechanically vented patients, because the breath-to-breath variation can be minimized. However, the evidence supporting use of IVC ultrasound in spontaneously breathing patients – which is largely our ED population – is questionable at best.
Bottom line:
- At extremes, IVC collapsibility – either very large or very small – may have some value in predicting volume status, however, IVC collapsibility has a wide range where it is clinically indeterminate in the spontaneously breathing patient.
- While measuring the respiratory variations in IVC seems simple, it should not be used as our sole basis for determining a patient’s fluid status
Take Home Points
- River’s trial end-points are not gospel.
- Lactate = Stress
- Utilize lactate as a screen in patients with suspected sepsis, but…
- Do not interpret it as a biomarker of hypoxia, but as a major protective component of the stress response that is also a strong predictor of mortality.
- MAP = one size may not fit all:
- Individualize your MAP targets: probably not doing any good by targeting a higher MAP unless they have chronic hypertension.
- CVP = Not worth it in isolation.
- Very unreliable, and does not reflect volume status.
- IVC U/S = no evidence in spontaneously breathing patients.
- Not the magical, easy, non-invasive end-point that we originally hoped, so you can’t use it as the ultimate end-point.
- If you’re seeing a collapse of anywhere from 30-50%, you could interpret that the patient may be fluid responsive, but more or less than that it is difficult to draw any conclusions.
Dr. Gauri Ghate is a 4th year Emergency Medicine resident at the University of Ottawa, with a special interest in resuscitation and critical care.
Edited by Dr. Shahbaz Syed, 4th year Emergency Medicine Resident, University of Ottawa.
References
1. Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39(2):165-228.
2. Marik PE, Baram M, Vahid B: Does the central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest 2008; 134:172–178
3. Rivers E, Nguyen B, Havstad S, et al: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345:1368 –1377
4. Serum lactate as a predictor of mortality in emergency department patients. 2005 May;45(5):524–8.
5. Jansen TC, van Bommel J, Schooderbeek FJ et al; LACTATE group: Early lactate-guided therapy in intensive care unit patients: A multicenter, open-label, randomized control trial. Am J Respir Crit Care Med 2010; 182:752-761
7. Asfar, Pierre, Ferhat Meziani, Jean-François Hamel, Fabien Grelon, Bruno Megarbane, Nadia Anguel, Jean-Paul Mira, et al. 2014. High versus low blood-pressure target in patients with septic shock. The New England journal of medicine, no. 17 (March 18). doi:10.1056/NEJMoa1312173.http://www.ncbi.nlm.nih.gov/pubmed/24635770.
8. Bourgoin, Aurélie, Marc Leone, Anne Delmas, Franck Garnier, Jacques Albanèse, and Claude Martin. 2005. Increasing mean arterial pressure in patients with septic shock: effects on oxygen variables and renal function. Critical care medicine, no. 4.http://www.ncbi.nlm.nih.gov/pubmed/15818105.
9. Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370(18):1683-93.
10. Peake SL, Delaney A, Bailey M, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371(16):1496-506.
11. Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-11.
12. Friedman G, De Backer D et al. Oxygen supply dependency can characterize septic shock. Int Care Med. 1998. 24: 118-123
13. Shapiro N, Howell M.D, et al. Serum lactate as a predictor mortality in Emergency Department Patients with Infection. Annals of Emergency Medicine. 2005;45(5)
14. Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-81.
15. Leone M., Asfar P. et al. Optimizing mean arterial pressure in septic shock: a critical reappraisal of the literature. Critical Care (2015) 19:101
16. Lanspa MJ, Grissom CK, Hirshberg EL, Jones JP, Brown SM. Applying dynamic parameters to predict hemodynamic response to volume expansion in spontaneously breathing patients with septic shock. Shock. 2013;39(2):155-60.
17. Muller L, Bobbia X, Toumi M, et al. Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: need for a cautious use. Crit Care. 2012;16(5):R188.
18. Garcia-alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18(5):503.
19. Marik PE, Bellomo R, Demla V. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Critical Care 2013 Mar 01;1(1):3.
20. Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3(1):12.