Summary
Methodology Score: 4/5
Usefulness Score: 3.5/5
Patel BK, et al. JAMA. 2016 Jun 14;315(22):2435-41
Full Article
Editorial: Unmasking a Role for Noninvasive Ventilation in Early Acute Respiratory Distress Syndrome. Beitler JR, et al. JAMA. 2016 Jun 14;315(22):2401-3.
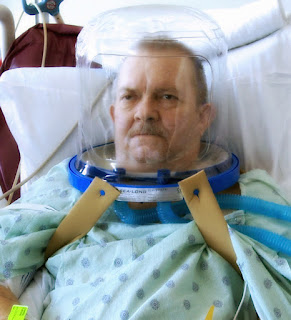
This single-centre, non-blinded RCT of ICU ARDS patients, found that noninvasive ventilation (NIV) delivered by a novel helmet, as compared to a standard NIV facemask, reduced intubations (18.2% vs. 61.5%; P <0.001) and hospital mortality (27.3% vs. 48.7%; P= 0.04). Despite its few methodological flaws, this study supports the existing literature on the advantage of oxygen delivered via helmet over facemask in avoiding intubation in ARDS, and future studies should focus on its impact in heart failure or hypercapneic respiratory failure.
By: Dr. Shannon Fernando