How many times a shift do you consider a urinary tract infection?
As Emergency physicians, you make and/or consider urinary tract infection (UTI) multiple times per shift.1 Actually, up to 5% of ED visits are related to genitourinary complaints.2-4 From simple cystitis, to pyelonephritis, and even life-threatening urosepsis, UTI’s and their wide spectrum of presentations are the MOST COMMON indication for empiric antibiotics. However, in 2014, the Centers for Disease Control and Prevention reported that antibiotic use in UTIs was avoidable 40% of the time.7
Why is this the case?
- First: UTIs remain a clinical entity fraught with dogma and practice variation. Everyone has different interpretations for the tests they order and thresholds for antibiotic prescription. 1,6,7
- Second: Urinalyses are often misinterpreted. 1,9
- Third: Urine cultures are ordered without appropriate indications. 1,9
- Lastly: Our ever-aging population makes UTI dx even more challenging. They often have a vague history, may not even be able to communicate for themselves, and have HIGH rates of ASYMPTOMATIC BACTERIURIA.
Why does this matter?
How we diagnose and manage UTIs in the ED has both individual, hospital and societal consequences. From the costs of unnecessary tests to the impact of inappropriate antibiotic treatment.10-12 How we diagnose UTIs matter. Yet most of our education and discussions around UTIs tends to focus on issues of antibiotic selection and less on the actual clinical diagnosis and applications of our urine tests.
So let’s get started! From the history to the physical, to test Interpretation and management, this blog post will hopefully become your ONE-STOP shop for most things relating to UTI’s.
Diagnosing a UTI
Step 1: Using History and Physical Exam: Is it an upper vs lower urinary tract infection?
This is important for two reasons, (1) by diagnosing a lower UTI we can manage a patient’s symptoms and prevent the evolution of the infection to an upper UTI; and (2) this distinction will result in different antibiotic choices and length of treatment.1 Unfortunately, a universally accepted gold standard on how to define and diagnose a UTI does not exist.12
As evident in the 2018 NICE guidelines, a 2018 position statement by Choosing Wisely Canada, the 2019 IDSA guidelines, the 2020 BC UTI Guidelines, and the 2021 EAU guidelines, the diagnosis of a UTI is primarily a clinical diagnosis not a laboratory one6,12-15. The urine tests we order may inform antibiotic management in specific situations, but rarely do they have important implications on the diagnosis of UTI in the ED.
In 2002, Bent and colleagues reviewed the accuracy and precision of history and physical examination for the diagnosis of UTI (refer to figure 1).16 Woman who previously had cystitis and are presenting with symptoms suggestive of another episode of UTI had a positive LR of 4. Symptoms like dysuria, frequency and hematuria individually have a LR ranging from 1.5-2.17
While the combination of symptoms like dysuria and frequency had a PPV around 50%, the absence of symptoms such as vaginal irritation, bleeding, or discharge; symptoms more in keeping with vaginitis or cervicitis, increases the PPV for UTI to over 90%. The combination of dysuria and frequency in the absence of vaginal discharge or irritation had a positive LR of 25.1,8,16-19

Figure 1: Likelihood Ratios of Signs and Symptoms of UTI1,8
Step 2: Using urine testing to help make the Dx
Use of urine tests to diagnose UTI’s is actually quite controversial. When compared head to head with history and physical exam, the urinalysis has limited added value. Admittingly, the quality of evidence around urinalysis is poor given the varied clinical definitions of UTI and ranges of clinical setting in which these studies were performed.
So let’s go through a few things
Midstream Samples
The midstream clean catch remains the gold standard for urine samples. But as many of the ED nurses know and the literature supports, this is often very difficult to obtain in a busy ED, particularly in our elderly and combative patients. A 2012 study showed that not only do we rarely provide appropriate instructions in urine collection but that patients rarely perform the essential steps even if they received and understood the directions correctly.22 A 2015 study by the same authors found that urine samples were often abnormal even in completely asymptomatic women regardless of technique (35% Leukocyte Esterase (LE)+ using the gold standard and 50% LE+ in the “non-clean” collection group).23 From the ED perspective the collection method doesn’t appear to impact our clinical use of urinalysis.
Urine Smell and Turbidity
The appearance and smell of urine is not reliable and susceptible to observer error. It is also often more reflective of a patient’s hydration status and urea concentration and less about an infection.1 In a study of 100 anonymous urine samples from female patients aged 18 to 50, the sensitivity of urine turbidity was 13%, specificity 95%, positive predictive value 44% and negative predictive value 86.3%.24-26
Urinalysis
Urine POCT and urinalysis has largely replaced microscopy because it is less expensive and more convenient, with comparable accuracy. There are many things reported on a urinalysis, but we really only care about two things – leukocyte esterase (LE) and nitrites.
Leukocyte Esterase: LE is an enzyme produced by neutrophils and reflects whole or lysed white blood cells in the urine. It’s an indicator of inflammation in the urinary tract or kidneys; but on its own, is not diagnostic of a UTI as many factors can cause false positives.
Nitrites: Nitrates in the urine are converted to nitrites in the presence of Gram-negative bacteria such as E.coli and Klebsiella. A positive nitrite test is a surrogate marker of bacteriuria; but again, Nitrites do not equate to UTI in isolation. Similarly, interpreting nitrites get muddled by several factors, most notably by the fact that bacteria like S. saprophyticus, which can make up 5-15% of our UTIs do not convert nitrates to nitrites.
The most cited testing statistics on the accuracy of dipstick urinalysis in the prediction of cystitis, originates from a 1991 meta-analysis by Hurlbut and Littenberg. In this analysis, they identified and summarized 51 studies (see Table 1).
It is important to remember that in this case we care more about positive predictive value (PPV) and the negative predictive value (NPV), since the sensitivity and specificity of a test are more relevant on a population level. PPV and NPV tell us the probability that a person who receives a positive test or negative test result have the disease. For UA, a PPV value of having both LE and nitrites present is 51% and the NPV is 88% (95% CI 84-92%) (refer to table 1). What that means is that if we base our diagnoses and treatment thresholds on urinalyses alone, we would theoretically overtreat 49% of our patients while missing up to 12% of cases.1,27-30
| Sn | Sp | PPV | NPV | |
| LE + | 77% (80-90%) | 54% (54-98%) | ||
| Nitrites + | 81% (32-81%) | 87% (83-93%) | ||
| LE + / Nitrites +: | 75% (67-100%) | 93% (67-98%) | ||
| LE+ and Nitrite + | 94% | 50% | 51% | 88% |
Table 1. Urinalysis Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value18-20,27
The results of the urinalysis won’t significantly change our post-test probability either. Utilizing the LR from Bent et al, a positive POCT test gave a +LR of 4 and a –LR of 0.3. So, if we have a patient with a very low pre-test probability, you are unlikely to treat that patient even if that UA was positive (refer to figure 2).
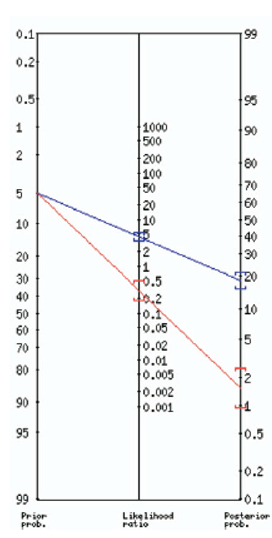
Figure 2. Fagan nomogram of post-test probability using urinalysis in a patient with a low probability of UTI (5%)
Nor would it impact your diagnosis in a patient with a high pre-test probability because a positive or negative UA will not change your post-test probability enough to change your decision, especially when you consider that a combination of UTI symptoms can provide a LR of almost 25 (refer to figure 3).
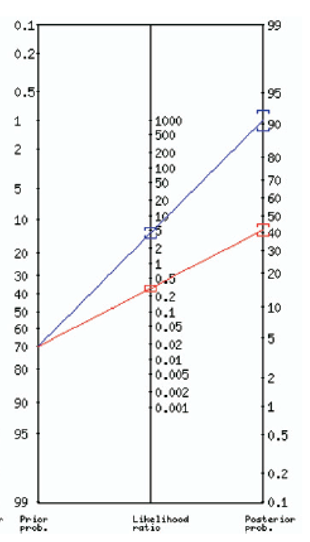
Figure 3. Fagan nomogram of post-test probability using urinalysis in a patient with a high probability of UTI (70%)
Provinces like British Columbia have proposed utilizing UA mainly in intermediate risk patients, characterized as females who were presenting atypically or with 1 or 2 symptoms of UTI. In these cases where your pre-test probability hovers around 50%, they suggest using the high specificity of 93% for LE and or Nitrites being present to rule in the diagnosis of UTI and treat. Alternatively, they recommend using the NPV 88% of both LE and nitrates being absent to rule out the diagnosis (refer to figure 4).13
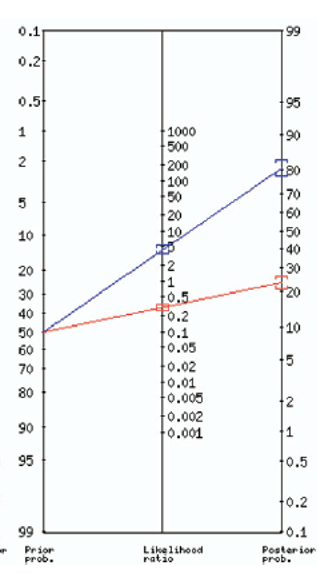
Figure 4. Fagan nomogram of post-test probability using urinalysis in a patient with a intermediate probability of UTI (50%)
Urine Microscopy
Urine Microscopy counts the type of cells, bacteria, casts, and crystals. For pyuria to correlate with appropriate bacteria concentrations, a cut-off of >10 WBC/mm3 is typically cited in the literature. While bacteria on microscopy is reflective of bacteriuria, it is not diagnostic of a UTI in isolation as it could reflect asymptomatic bacteriuria or contamination (Sn of 91% (60-100%), Sp 50% (40-100%), PPV 67%, NPV 83%).31,32
Microscopy’s use in UTI is often questioned given the convenience, testing comparability and cost of UA. In Montreal, and BC altogether, microscopy has been removed from their standard protocols citing that although urine microscopy is automated it’s still often reviewed by a technician or nephrologists 10–20% time, meaning it remains highly operator dependent. It’s expensive and time consuming compared to other urine tests and there are no universal reference standards, which leads to poor sensitivities. One study by Chen et al., showed that it’s removal from standard protocols resulted in a 95% reduction in the number of urine microscopies performed at their sites (refer to figure 5). The largest reduction of which came from the ED and outpatient setting. Just as important, a follow up survey showed that 88% of physicians considered the change to have no effect on their clinical practice at 1- and 5-years post. 47,000 urine microscopies were eliminated annually which translated to over $200,000 a year.33
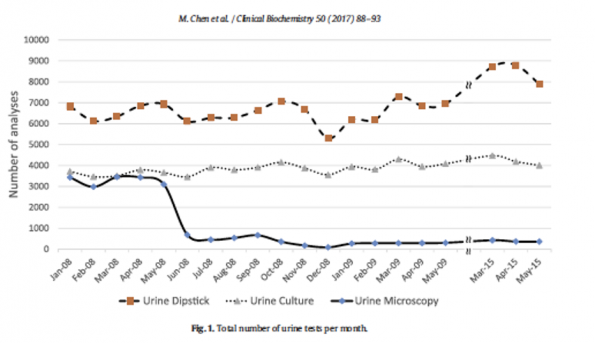
Figure 5. Elimination of urine microscopies in Montreal, Quebec Canada.33
Urine Culture
The criteria of what defines a positive urine culture is debated in the literature. The traditional definition and what most laboratories define as a positive result is a colony count ≥ 105 CFU/mL which has a high sensitivity at 95%, but low specificity at 85%. Using quantitative cut offs are imperfect however, with some woman having symptomatic UTIs and uropathogen counts documented as low as 102.13,15,18,19
The consensus is that urine cultures are not necessary to make the diagnosis of UTI in patients with simple cystitis. Nor will it help in the management of the majority of our lower UTIs in the ED as over 75-90% of infections are caused by E.Coli. In a retrospective study looking at the outpatient management of simple cystitis at a family medicine clinic, the follow up rate with or without culture was not statistically different (OR 1.11 (95% CI 0.65-1.90)).34 With one study showing that the number needed to test to prevent one follow up visit for a resistant uropathogen at 23.35
Step 3: Take Home Point #1 – Urine tests do not provide the dx of UTI.
Urine testing is unlikely to change our diagnosis or management of a simple lower UTI. Simple cystitis has minimal risk of progression to tissue invasion or sepsis, with a large proportion of lower UTIs being benign and self-limiting.6 Several randomized control studies have shown rates of symptom resolution in over 50% of patients when treated with placebo or ibuprofen alone.36-38 Furthermore, studies in Family Medicine using telephone consultation for cystitis have shown significant reduction in testing rates in the outpatient setting while having no changes in rates of bounce backs for cystitis, STI, or pyelonephritis.
Urine tests should be reserved for when the history suggests that antibiotic choice may impact management. These indications include, but are not limited to; males, the immunosuppressed, patients with a history of multiple drug allergies, risk of an antibiotic resistance organism (i.e., recent antibiotic use or hospitalization, travel), multiple courses of antimicrobial therapy, recurrent UTI (>3 per year – this is expert opinion) and lastly suspicion for upper UTI or urosepsis.1,13
UTIs are a clinical diagnosis. Healthy non-pregnant females with classic cystitis do not require routine urine testing in the ED.
Asymptomatic Bacteriuria (ASB)
There is a large proportion of ED patients without any signs or symptoms of a UTI where urinary tract infections are questioned because a urine test shows evidence of pyuria or bacteriuria. This is known as “asymptomatic bacteriuria” and is defined as the presence of bacteria in the bladder or urine without symptoms pertaining to a UTI. It reflects a state of colonization but NOT an infection. Bladders are not always sterile and finding bacteria in the urine may be normal, with rates as high as 8% in young women and up to 40-50% in our male and female Long-Term Care (LTC) residents.41,42
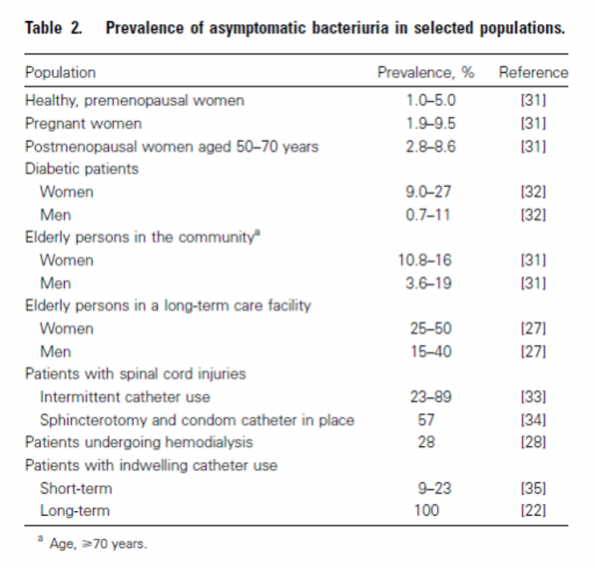
Figure 6. Prevalence of asymptomatic bacteriuria.41,42
Several studies, Cochrane reviews, the 2010 IDSA guidelines, and choosing widely Canada have reviewed and provided several approaches to the management of ASB. The Cole’s notes version to this mountain of evidence is that – unless the patient is pregnant or undergoing invasive urologic procedures, screening, and treating asymptomatic bacteriuria has no clinical or mortality benefit to our patients.
Asymptomatic Bacteriuria in our older ED population
As documented throughout the literature older patients are more frequently diagnosed with and treated for UTI despite the absence of symptoms with one study showing almost 50% of prescriptions were given to LTC residents in the absence of UTI symptoms.43
As ED physicians we over investigate, over-interpret and subsequently over-treat patients with ASB, leading to increased side effects (i.e., C. difficile diarrhea, MRSA) and antimicrobial resistance, all in the absence of a morbidity or mortality benefit.9,46 There is also evidence that we may be placing these patients at risk by altering their protective microbiome. In patients with spinal cord injuries, when a nonvirulent E.Coli strain was instilled into patient’s bladders, in comparison to normal saline, the treatment group had fewer symptomatic UTIs during the subsequent year (>1 episode of UTI: 29% colonized with E. coli compared to 70% placebo (P = 0.049); average number of UTI/patient-year: 0.50 colonized with E.coli vs 1.18 Placebo (P <0.02)).47 Similarly when young women with asymptomatic bacteriuria and previous symptomatic infections were randomized to antibiotic treatment or placebo, the antibiotic group had more symptomatic recurrences during the 1 year follow up (6 month recurrence if treated with antibiotics: RR 1.31 (95% CI 1.21-1.42), P < 0.0001); 12 month recurrence if treated with antibiotics RR 3.17; 95% CI, 2.55-3.90, (P < .0001)).48
To better identify what is ASB, we need to better delineate what symptoms are not suggestive of UTI in isolation. As per the 2019 IDSA, the 2020 BC UTI guidelines, and the Ottawa Infectious Disease department, symptoms or signs that should not be considered indicative of a UTI in isolation include: falls, chronic nocturia, chronic incontinence, cloudy or malodorous urine, changes in appetite, aggression, wandering, general fatigue or malaise. Most importantly, altered mental status and delirium are not to be considered secondary to UTI in the absence of urinary signs or symptoms.12,13
ASB, UTI and delirium are clinical entities ripe with dogma and practice variation. A 2021 Ottawa based survey reviewed investigation and treatment patterns of asymptomatic bacteriuria in delirious patients over the age of 65 in 297 Canadian Physicians, 50% of whom were ED physicians. In this study, there was a propensity for testing delirious patients for UTI causes, with 79% requesting urinalysis and 52% ordering a urine culture. If bacteriuria was found in delirious but symptomatically free and systemically well patients, 38% would still treat immediately, 34% would wait for culture, 14% would treat if no other cause was found for delirium, and only 14% would refrain from giving antibiotics altogether.49
Many patients presenting to our ED with confusion especially from LTC facilities present with limited, if any, history. We often can’t delineate what is a new symptom versus what is chronic and trying to obtain a reliable history is often close to impossible. Getting urine specimens, however, can be relatively easy especially from catheterized patients, so it is not surprising that bacteriuria has become a scapegoat for delirium. The treatment with antibiotics is easy, the family feels reassured, and the physician gets their diagnosis. The challenge with this practice, however, is the patient might have normalized without antibiotics. As shown in a prospective cohort study of 432 hospitalized older patients, 70% of the 62 patients diagnosed with delirium had spontaneous resolution of their symptoms in 1 day.50
As emergency physicians our diagnoses and treatment have downstream impacts on patient care both inside and outside of the ED, whether it is the propagation of a diagnosis or treatment on the ward, or the creation of expectations by families around bacteriuria and antibiotics in delirium, how we label and diagnose UTIs matters.
Two recent systematic reviews of over 22 studies could not make a link between delirium and ASB, nor to simple cystitis.51,52 The studies that do suggest a causal relationship between UTI and delirium, are often observational studies, with inadequate sample sizes, lack a clear definition of UTI and delirium, do not evaluate for other causes of delirium, and often never adjust for age or comorbidities.53,54
Currently, insufficient evidence exists to show that asymptomatic bacteriuria is causative of delirium and as ED physicians we risk making future diagnostic errors through premature closure. Healthy skepticism needs to be employed before labeling delirium in a patient without clear evidence of urinary symptoms as secondary to bacteriuria.
Take Home Point # 2: Insufficient evidence exists to show that asymptomatic bacteriuria is causative of delirium
Not surprisingly, the diagnosis of ASB often leads to unnecessary investigations and treatment. In 2009, a retrospective review of 335 confused patients with symptomatic or asymptomatic bacteriuria showed that confusion (57%) was the most common indication for sending a urine culture. Among the 67 patients without focal UTI symptoms and confusion, 64% were treated with antibiotics yet no mortality benefit was found between these groups (P=0.36).56
A prospective cohort study of 343 delirious in-patients aged 70 years or older were screened every 2 days for delirium both during hospitalization and following discharge. 27% of delirious patients without focal signs of UTI were treated with antibiotics. Compared to other delirious inpatients, treatment for asymptomatic UTI was associated with worst functional recovery defined as death, new permanent institutionalization, or decreased ability to perform basic activities of daily living (RR 1.30, 95% CI: 1.14-1.48). Moreover, those in the ASB group treated with antibiotics had greater risks of C. difficile infections (OR 2.45, 95% CI: 0.86-6.96).57
Pinnell and colleagues looked at adults over the age of 65 presenting to the Ottawa ED with confusion and the rates at which they received urine tests, UTI diagnoses and antibiotics. Out of a group of 499 confused patients, 324 received urine tests of which only 69 patients had UTI symptoms. In the subgroup of patients without UTI symptoms, fever, or other ED infectious disease diagnoses, 58% received urine tests, 8% received a diagnosis of UTI, and 18% received antibiotics. Those who had been given antibiotics in the absence of symptoms had increased rates of admission (OR 2.7 (95% CI 1.5–4.9) and higher rates of 30-day (OR 3.8 (95% CI 1.1-12.2) and six-month mortality (2.7 (95% CI 1.03-6.6) even after adjusting for age and sex.58
When a universal UTI criterion without delirium or altered mental status was applied in a cluster randomised control trial of 24 nursing homes in Canada and the United States, in comparison to standard care, there was no difference in rates of hospitalization or death. There was however a significant reduction in antibiotic treatment for UTIs (1.17 vs 1.59 antibiotic courses, weighted mean difference −0.49, 95% CI −0.93 to −0.06).59
There is little evidence to suggest that delirious patients with bacteriuria without UTI symptoms do better with antibiotic treatment. Nor is there evidence of benefit in patients with baseline cognitive or functional deficits. In 4 randomized control trials of community dwelling and LTC residents, 70% of whom were labeled as having dementia and 30% with severely impaired mental status (otherwise known as an MMSE of <12), treatment of new delirium with antibiotics for ASB showed no benefit in mortality or infectious morbidity.12
It is for this reason that the 2019 IDSA guidelines recommend that older patients with functional and or cognitive impairment with bacteriuria and delirium without local or systemic signs of UTI do not require routine antimicrobial treatment (strong recommendation, low-quality evidence).12 These recommendations have already been incorporated into BC’s 2020 provincial UTI guidelines. Importantly, this guideline also recommends deferring urine tests completely in patients presenting with new delirium and non-urinary symptoms (refer to figure 7).
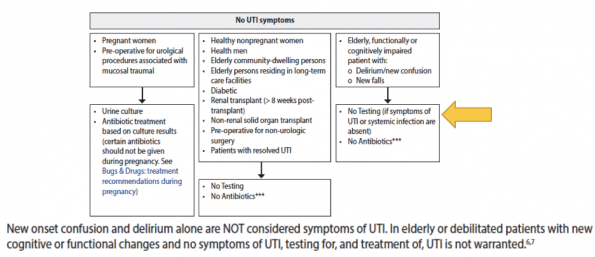
Figure 7. BC 2020 Recommended Guidelines to ASB including patients with functional or cognitive impairment.13
In accordance with the 2018 AMMI Canadian position statement, this protocol proposes a trial of observation or rehydration for 24 -48 hours instead of urine testing. The focus instead is on good delirium management while optimizing the patient and treating other common causes of delirium, such as dehydration, hypoglycemia, and polypharmacy (refer to figure 8.)
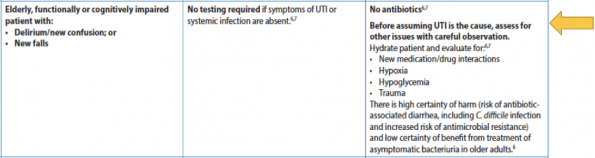
Figure 8. BC 2020 Recommended Guidelines to ASB in older patients, those with functional or cognitive impairment.13
Multiple initiatives in Canada have been implemented to address inappropriate use of antibiotics in asymptomatic bacteriuria, including the campaign “Symptom free Pee: Let it be” by the Association of Medical Microbiology and Infectious Disease Canada. Conservative measures and symptom management is also supported by Public Health Ontario (refer to Figure 9) and the Infectious Disease Department of Ottawa (refer to Figure 10).
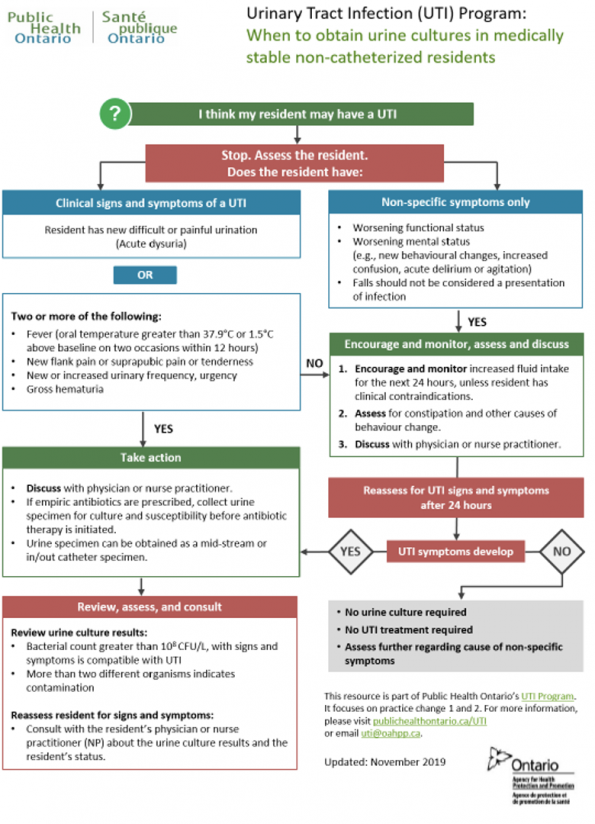
Figure 9. Public Health Ontario UTI Program and recommendations in patients with nonspecific symptoms.

Figure 10. Antimicrobial Stewardship Program recommendations for when to send urine cultures at the Ottawa Hospital
Take Home Point Number 3: In non-catheterized patients with new delirium and bacteriuria without symptoms or systemic signs of UTI, whether they have baseline functional or cognitive impairment or not, a period of observation for 24-48 hours is recommended not antibiotics.
As emergency physicians, how we diagnose UTIs in the ED has both individual, hospital and societal consequences. We are at the vanguard of how these patients are diagnosed and subsequently managed on the wards, in the LTC facilities, and at home. How we label, diagnose, and treat UTIs and ASB, especially in the elderly, matters. So, own it!
References
- Helman, A, Morgenstern, J, Morris, A. UTI Myths and Misconceptions. Emergency Medicine Cases. https://emergencymedicinecases.com/uti-myths-misconceptions/. Accessed Nov.1/21
- Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002;113(Suppl):5S–13S.
- Nawar EW, Niska RW, Xu J. National Hospital Ambulatory Medical Care Survey: 2005 emergency department summary. Adv Data 2007;386:1–32.
- Morgan MG, McKenzie H. Controversies in the laboratory diagnosis of community-acquired urinary tract infection. Eur J Clin Microbiol Infect Dis 1993;12(7):491-504.
- Schulz, L., Hoffman, R. J., Pothof, J., & Fox, B. (2016). Top Ten Myths Regarding the Diagnosis and Treatment of Urinary Tract Infections. Journal of Emergency Medicine, 51(1), 25–30. https://doi.org/10.1016/j.jemermed.2016.02.009
- Gupta, K., Hooton, T. M., Naber, K. G., Wullt, B., Colgan, R., Miller, L. G., Moran, G. J., Nicolle, L. E., Raz, R., Schaeffer, A. J., & Soper, D. E. (2011). International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinical Infectious Diseases, 52(5), 103–120. https://doi.org/10.1093/cid/ciq257
- Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep 2014;63:194–200.
- Justin Morgenstern. UTI: More than you ever wanted to know, First10EM, 2017. Available at: https://doi.org/10.51684/FIRS.4470. Accessed Nov.7/21
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. Am J Med 1987;83(1):27-33.
- Harding GK, Zhanel GG, Nicolle LE, Cheang M. Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med 2002;347(20):1576-83.
- Health Protection Agency, British Infection Association. Management of infection guidance for primary care for consultation and local adaptation. London: HPA; 2010. Available from http://www.hpa.org.uk/webc/HPAwebFile/ HPAweb_C/1279888711402: [Accessed. 26 Jun. 2012.]
- Nicolle, L. E., Gupta, K., Bradley, S. F., Colgan, R., DeMuri, G. P., Drekonja, D., Eckert, L. O., Geerlings, S. E., Köves, B., Hooton, T. M., Juthani-Mehta, M., Knight, S. L., Saint, S., Schaeffer, A. J., Trautner, B., Wullt, B., & Siemieniuk, R. (2019). Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 68(10), E83–E75. https://doi.org/10.1093/cid/ciy1121
- Recommendations, K. (2020). Urinary Tract Infections in the Primary Care Setting – Investigation Tests for UTI. 10(July). https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/urinary-tract-infections?keyword=urinalysis#key_recommendations%0Afile:///C:/Users/mar04/Desktop/Nueva carpeta/urinalysis.pdf
- Leis, J. A., Hatchette, T., Ciccotelli, W., Daley, P., Goneau, L., Gregson, D., Kulkarni, S., Loo, V., Lagace-Wiens, P., Lowe, C. F., Matukas, L., Roscoe, D., Rubin, E., & Gold, W. L. (2018). Choosing wisely canada—top five list in medical microbiology: An official position statement of the association of medical microbiology and infectious disease (AMMI) canada. Journal of the Association of Medical Microbiology and Infectious Disease Canada, 3(2), 61–70. https://doi.org/10.3138/jammi.2018.02.08
- National Institute for Health and Care Excellence. (2018). Urinary tract infection (lower): antimicrobial prescribing. Nice, November 2018, 1–29. https://www.nice.org.uk/guidance/ng109
- Bent S, Nallamothu BK, Simel D, et al. Does this woman have an acute uncomplicated urinary tract infection? JAMA 2002;287:2701–10.
- Aubin C. Evidence-based emergency medicine/rational clinical examination abstract. Does this woman have an acute uncomplicated urinary tract infection? Annals of emergency medicine. 49(1):106-8. 2007
- Lane DR, Takhar SS. Diagnosis and management of urinary tract infection and pyelonephritis. Emergency medicine clinics of North America. 29(3):539-52. 2011.
- Norris DL, Young JD. Urinary tract infections: diagnosis and management in the emergency department. Emergency medicine clinics of North America. 26(2):413-30, ix. 2008.
- Cadogan, M. (2020). Dipstick urinalysis • LITFL • CCC Investigations. In Life In The Fastlane. https://litfl.com/dipstick-urinalysis/
- Long, B., & Koyfman, A. (n.d.). The Lowly Urinalysis: How to Avoid Common Pitfalls. In Epmonthly.Com. https://epmonthly.com/article/lowly-urinalysis-avoid-common-pitfalls/
- Frazee BW, Frausto K, Cisse B, White DE, Alter H. Urine collection in the emergency department: what really happens in there? West J Emerg Med. 2012 Nov;13(5):401-5. doi: 10.581/westjem.2012.1.6855.
- Frazee BW, Enriquez K, Ng V, Alter H. Abnormal urinalysis results are common, regardless of specimen collection technique, in women without urinary tract infections. J Emerg Med. 2015 Jun;48(6):706-11. doi: 10.1016/j.jemermed.2015.02.020. Epub 2015 Apr 1. PMID: 25841289.
- Foley A, French L. Urine clarity inaccurate to rule out urinary tractinfection in women. J Am Board Fam Med 2011;24:474–5.
- Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol 2001;22:316–21.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643–54.
- Hurlbut TA, Littenberg B. The diagnostic accuracy of rapid dipstick tests to predict urinary tract infection. Am J Clin Pathol 1991;96:582–8.
- Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis 2004;38:1150–8.
- Rehmani R. Accuracy of urine dipstick to predict urinary tract infections in an emergency department. J Ayub Med Coll Abbottabad 2004;16(1):4–7.
- Pappas PG. Laboratory in the diagnosis and management of urinary tract infections. Med Clin North Am 1991;75:313–25.
- Blum RN, Wright RA. Detection of pyuria and bacteriuria in symptomatic ambulatory women. J Gen Int Med 1992;7(2):140- 4.
- Jenkins RD, Fenn JP, Matsen JM. Review of urine microscopy for bacteriuria. JAMA 1986;255(24):3397-403. (Med. Clin. N.A. 75: 313, 1991).
- Chen, M., Eintracht, S., & MacNamara, E. (2017). Successful protocol for eliminating excessive urine microscopies: Quality improvement and cost savings with physician support. Clinical Biochemistry, 50(1–2), 88–93. https://doi.org/10.1016/j.clinbiochem.2016.06.016
- Long, B., & Koyfman, A. (n.d.). The Lowly Urinalysis: How to Avoid Common Pitfalls. In Epmonthly.Com. https://epmonthly.com/article/lowly-urinalysis-avoid-common-pitfalls/
- McNulty CA, Richards J, Livermore DM, Little P, Charlett A, Freeman E, Harvey I, Thomas M. Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J Antimicrob Chemother. 2006 Nov;58(5):1000-8. doi: 10.1093/jac/dkl368. Epub 2006 Sep 23. PMID: 16998209.
- Christiaens, T. C. M., De Meyere, M., Verschraegen, G., Peersman, W., Heytens, S., & De Maeseneer, J. M. (2002). Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. British Journal of General Practice, 52(482), 729 LP – 734. http://bjgp.org/content/52/482/729.abstract
- Kronenberg, Andreas, et al. “Symptomatic Treatment of Uncomplicated Lower Urinary Tract Infections in the Ambulatory Setting: Randomised, Double Blind Trial.” BMJ, vol. 359, BMJ Publishing Group LTD, 2017, pp. j4784–j4784, https://doi.org/10.1136/bmj.j4784.
- Gágyor I, Bleidorn J, Kochen MM, Schmiemann G, Wegscheider K, Hummers-Pradier E. Ibuprofen versus fosfomycin for uncomplicated urinary tract infection in women: randomised controlled trial. BMJ. 2015 Dec 23;351:h6544. doi: 10.1136/bmj.h6544. PMID: 26698878; PMCID: PMC4688879.
- Barry, H. C., et al. “A Randomized Controlled Trial of Telephone Management of Suspected Urinary Tract Infections in Women.” The Journal of Family Practice, vol. 50, no. 7, Frontline Medical Communications Inc, 2001, pp. 589–94.
- Brusch J. Urinary Tract Infection in Males. Medscape. 2016. Accessed Nov.7/21
- Nicolle LE, Gupta K, Bradley SF, Colgan R, DeMuri GP, Drekonja D, et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019 Mar 21
- Nicolle LE. Asymptomatic Bacteriuria in the Elderly. Infect Dis Clin North Am. 1997 Sep 1;11(3):647–62
- Woodford HJ, George J. Diagnosis and management of urinary tract infection in hospitalized older people. J Am Geriatr Soc. 2009;57(1):107–14.
- Phillips CD, Adepoju O, Stone N, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012;12(1):73–80.
- Gordon LB, Waxman MJ, Ragsdale L, et al. Overtreatment of presumed urinary tract infection in older women presenting to the emergency department. J Am Geriatr Soc. 2013;61(5):788–92.
- Woodford HJ, George J. Diagnosis and management of urinary tract infection in hospitalized older people. J Am Geriatr Soc. 2009 Jan;57(1):107-14. doi: 10.1111/j.1532-5415.2008.02073.x. Epub 2008 Nov 14. PMID: 19054190.
- Darouiche RO, Green BG, Donovan WH, Chen D, Schwartz M, Merritt J, Mendez M, Hull RA. Multicenter randomized controlled trial of bacterial interference for prevention of urinary tract infection in patients with neurogenic bladder. Urology. 2011 Aug;78(2):341-6. doi: 10.1016/j.urology.2011.03.062. Epub 2011 Jun 17. PMID: 21683991.
- Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, D’Elia C, Malossini G, Boddi V, Bartoletti R. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis. 2012 Sep;55(6):771-7. doi: 10.1093/cid/cis534. Epub 2012 Jun 7. PMID: 22677710.
- Laguë, A., Boucher, V., Joo, P.et al. Investigation and treatment of asymptomatic bacteriuria in older patients with delirium: a cross-sectional survey of Canadian physicians. Can J Emerg Med 24, 61–67 (2022). https://doi.org/10.1007/s43678-021-00148-1
- Rudberg MA, Pompei P, Foreman MD, Ross RE, Cassel CK. The natural history of delirium in older hospitalized patients: a syndrome of heterogeneity. Age Ageing. 1997;26:169-174.
- Balogun SA, Philbrick JT. Delirium, a symptom of UTI in the elderly: fact or fable? A systematic review. J Am Med Dir Assoc. 2013;14(3):B21.
- Mayne S, Bowden A, Sundvall PD, et al. The scientific evidence for a potential link between confusion and urinary tract infection in the elderly is still confusing – A systematic literature review. BMC Geriatr. 2019;19(1):1–5.
- Juthani-Mehta M, Quagliarello V, Perrelli E, Towle V, Van Ness PH, Tinetti M. Clinical features to identify urinary tract infection in nursing home residents: a cohort study. J Am Geriatr Soc 2009; 57:963–70.
- Bhattacharya B, Maung A, Barre K, Maerz L, Rodriguez-Davalos MI, Schilsky M, Mulligan DC, Davis KA. Postoperative delirium is associated with increased intensive care unit and hospital length of stays after liver transplantation. J Surg Res. 2017 Jan;207:223-228. doi: 10.1016/j.jss.2016.08.084. Epub 2016 Sep 2. PMID: 27979481.
- Sundvall PD, Ulleryd P, Gunnarsson RK. Urine culture doubtful in determining etiology of diffuse symptoms among elderly individuals: a cross-sectional study of 32 nursing homes. BMC Fam Pract. 2011 May 19;12:36. doi: 10.1186/1471-2296-12-36. PMID: 21592413; PMCID: PMC3142216.
- Silver, S. A., Baillie, L., & Simor, A. E. (2009). Positive urine cultures: A major cause of inappropriate antimicrobial use in hospitals?.The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale, 20(4), 107–111. https://doi.org/10.1155/2009/702545
- Dasgupta M, Brymer C, Elsayed S. Treatment of asymptomatic UTI in older delirious medical in-patients: A prospective cohort study. Arch Gerontol Geriatr. 2017 Sep;72:127-134. doi: 10.1016/j.archger.2017.05.010. Epub 2017 May 31. PMID: 28624753.
- Pinnell RAM, Ramsay T, Wang H, Joo P. Urinary Tract Infection Investigation and Treatment in Older Adults Presenting to the Emergency Department with Confusion: a Health Record Review of Local Practice Patterns. Can Geriatr J. 2021 Dec 1;24(4):341-350. doi: 10.5770/cgj.24.518. PMID: 34912489; PMCID: PMC8629500.
- Loeb M, Brazil K, Lohfeld L, McGeer A, Simor A, Stevenson K, Zoutman D, Smith S, Liu X, Walter SD. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ. 2005 Sep 24;331(7518):669. doi: 10.1136/bmj.38602.586343.55. Epub 2005 Sep 8. PMID: 16150741; PMCID: PMC1226247.



Trackbacks/Pingbacks