Do you fumble using fundoscopy? Can’t find the ophthalmoscope in your department when you need to? In this post, we will show you how to refine your ocular PoCUS skills to help you manage those eye cases on your next shift.
First, let’s start with some basics. You want to throw on a Tegaderm over the eye with the eyelid closed to avoid getting any jelly in the eye. Have the patient lie flat or at 45 degrees. Use the linear probe, ideally set to an “ophthalmic” or “small parts” setting to minimize any damage to the eye from heat or mechanical effects of the ultrasound[1]. You may need to use a generous amount of gel to create adequate contact between the curved eye and the straight probe.Apply GENTLE pressure – not too much as this can be uncomfortable for your patient. Then, start scanning in the transverse and sagittal planes. There is one important caveat – if you suspect globe rupture, you should not perform ocular ultrasound as you don’t want to apply any pressure to the eye. For more information on how to scan the eye, visit the procedure handout here[2].
Here’s how the basic anatomy of the eye should look under POCUS:
Case 1: Papilledema
A 21-year-old female presents with a 2-week history of a throbbing bitemporal headache. Her headache has been constant, low grade, worse in the morning. There’s been only minimal relief with ibuprofen and Tylenol. She also reports a 1-week history of non-specific blurring of vision in both eyes and no loss of vision. You’re thinking this patient may have Idiopathic Intracranial Hypertension (IIH) and want to use your POCUS skills to see if she has papilledema – a marker of increased intracranial pressure. We place the linear probe onto her eye and this is what we see:

Here, you see a widened optic nerve sheath diameter (Photo taken from https://coreem.net/core/ocular-ultrasound/)
The optic nerve sheath diameter (ONSD) is used as a surrogate of increased intracranial pressure. The optic nerve sheath surrounds the optic nerve and communicates directly with the intracranial space – so if the intracranial pressure is increased, the optic nerve sheath diameter increases. We can measure the optic nerve sheath diameter at 3mm distal to the posterior aspect of the globe. Although there is some controversy to the exact cut off for the optic nerve sheath diameter, in general, for adults an ONSD ≤ 5mm is normal[3],[4],[5]. Many studies use a threshold of between 5-6mm as abnormal. One recent meta-analysis found that highest sensitivity is using an ONSD cutoff of ≥ 6 mm[6]
Here is a look at what a normal optic nerve sheath diameter looks like:

Normal Optic Nerve Sheath Diameter (Photo taken from Dr. Mandy Peach http://www.thepocusatlas.com/orbital)
Another finding you may see on POCUS in a patient with increased intracranial pressure (ICP) is optic disc elevation (ODE), as shown here:
Optic disc elevation is a bulging of the optic disc into the posterior chamber, and is the sonographic equivalent of papilledema. There is also some controversy here as to what the normal value is – either a cut-off of <0.6mm or < 1mm as normal. A recent pediatric study suggested the optimal cutoff is 0.66mm[7]. Using a cut-off value of 0.6mm may increase sensitivity as compared to using a cut-off of 1mm [8].
Although both ONSD and ODE are usually used as a marker of increased ICP, it’s important to remember that both are also seen with other entities such as optic neuritis, multiple sclerosis, malignancies, and many inflammatory and infiltrative processes such as sarcoidosis or lymphoma, which may result in false positives[9]. Common causes of “pseudopapilledema” include optic disc drusen and inflammatory processes[10]. A patient with chronically elevated ICP, like in a patient with IIH, should exhibit both a dilated optic nerve sheath as well as optic disc swelling. An acute elevation in ICP, such as an intracranial hemorrhage, may not demonstrate disc swelling as it may take hours to days to develop[11].
Take Home Point – POCUS can be an accurate way to look for signs of increased intracranial pressure, however a normal optic nerve by no means excludes life-threatening brain pathology. ICP estimation is merely one more piece of information to be integrated with clinical context, and it may help point you in the right direction.
Case 2: Retinal Detachment
A 61-year-old male presents with loss of vision in his left eye that began yesterday and has become progressively worse. He describes the sensation that a curtain come down over his affected eye. This curtain is not yet affecting his central vision. His visual acuity testing shows OD 20/25 and OS 20/30. Examination of visual fields detects a visual field defect in the left eye in the superior aspect of his vision.
You place the probe on his eye, and you see this:
Retinal Detachment (Photo taken from Dr. Rajiv Thavanathan, The Ottawa Hospital)
Here we see a thick, hyperechoic (ie. bright white) membrane lifted off of the posterior surface of the globe that is tethered to the optic nerve. If the retinal detachment is small, it may be adherent to the posterior wall of the eye and may not move much with eye movement, which may make it more difficult to detect. You should scan the entire eye in multiple axes to look for a small retinal detachment.
If the macula is already detached meaning “macula off”, central visual acuity is lost and this is typically permanent. If the macula is still on meaning “macula on”, central visual acuity is preserved as in this patient’s presentation, and this is a real emergency that we can prevent from worsening.
Take Home Point – In patients with retinal detachment, the retina will appear as a thick hyperechoic membrane that is attached to the back of the eye and is tethered to the optic nerve.
Case 3 – Vitreous Detachment vs Vitreous Hemorrhage
A 60-year-old female presents to the ED with flashing lights and floaters in the left eye x 3 days. She describes the floaters as “large and stringy”, and the flashing lights occurring intermittently.
You may be thinking this patient may have a posterior vitreous detachment or a vitreous hemorrhage. Here, you can use your POCUS skills to help differentiate between the two pathologies.
Vitreous Detachment
Posterior vitreous detachment occurs when the posterior vitreous body separates from the posterior portion of the retina. When visualized with POCUS, the hyperechoic membrane that is lifted off the posterior wall of the eye is thinner than seen in the previous case of retinal detachment. In fact, it may only be seen at higher gain settings. This thinner membrane is not tethered to the optic nerve. Instead, it moves freely with eye movements.
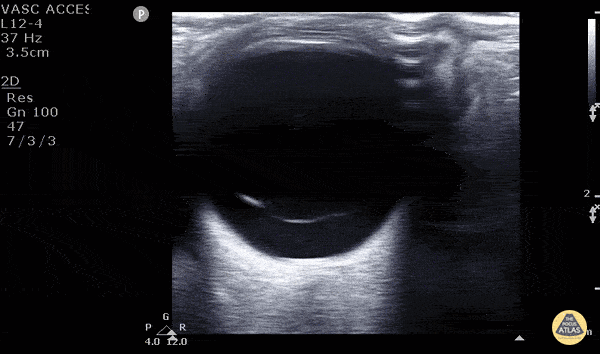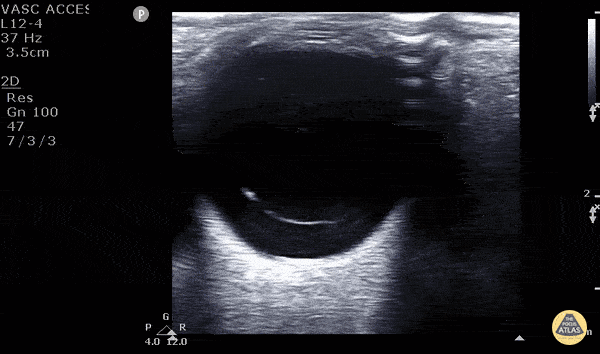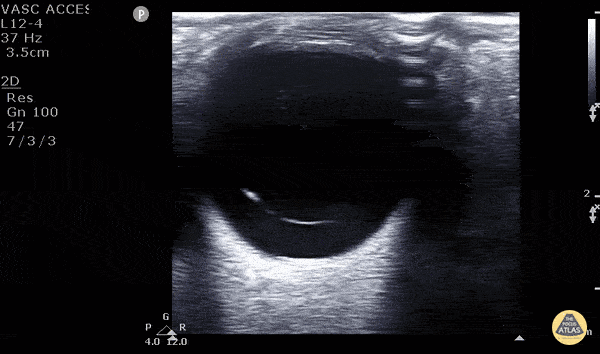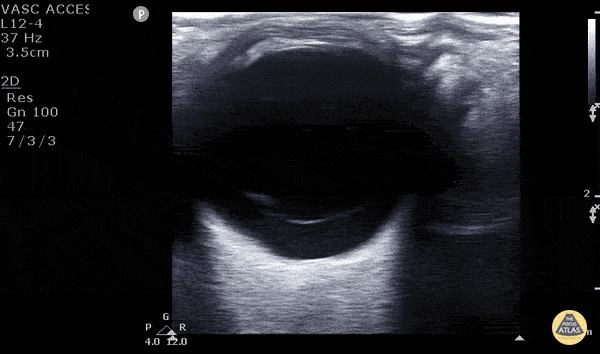
Vitreous detachment (Photo taken from Dr. Matt Riscinti and Dr. Bryan Flores, http://www.thepocusatlas.com/orbital)
Remember, the distinguishing POCUS features that differentiate vitreous detachment from retinal detachment: In vitreous the membrane is thin and freely moves with eye movements and may only be seen at higher gain settings. In retinal detachment, the membrane is thick and is tethered to the optic nerve.
Vitreous Hemorrhage
Patients with flashes and floaters may also have a vitreous hemorrhage which presents similarly with flashes and floaters. Vitreous hemorrhage occurs when extravasated blood is inside or around the vitreous humor of the eye. On POCUS, vitreous hemorrhage looks like echogenic material in the posterior chamber that is quite mobile. With eye movement, you may see something that has been described as a “washing machine sign”.
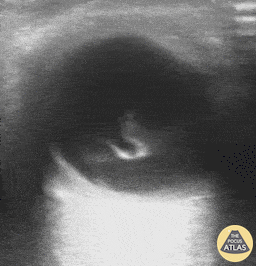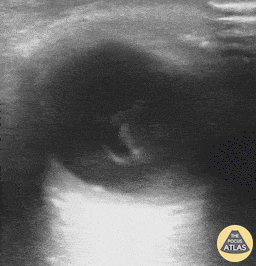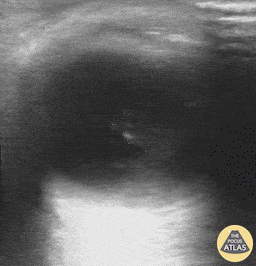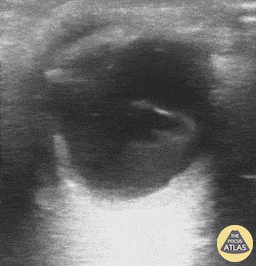
Vitreous Hemorrhage (Photo taken from Dr. Richard Cunningham, http://www.thepocusatlas.com/orbital)
Take Home Point – In patients with new onset flashes and floaters, use your ocular POCUS to see if there is evidence of a vitreous detachment (a thin membrane that moves freely and is not tethered to the optic nerve) and/or a vitreous hemorrhage (echogenic material in the posterior chamber that moves/spins with eye movement).
How good are emergency medicine physicians and residents at picking up these pathologies on POCUS? Fairly good! For retinal detachment, one meta-analysis found that POCUS had +LR ~25 and –LR 0.06[12]. We’re most sensitive at picking up retinal detachments, and less sensitive at picking up vitreous hemorrhage and vitreous detachment[13]. Small retinal tears (as opposed to retinal detachment) may also be difficult to exclude based on POCUS. If a patient presents with new visual symptoms of flashes/floaters/loss of vision, use your ocular POCUS skills to see if you can identify one of these pathologies recognizing that these patients should still be seen urgently by an ophthalmologist even if the ocular POCUS is normal.
Case 4 – Central Retinal Artery Occlusion
An 80-year-old male presents to the ED with 6 hours of acute, atraumatic, painless vision loss. He describes a sudden loss of vision that occurred while he was watching TV. His visual acuity testing shows OD 20/20 and OS movement of light.
Many of the clinical signs of CRAO (eg. pale retina, retinal edema, cherry red spot, attenuated arteriole) can be difficult to appreciate with non-dilated fundoscopy in the ED.
The POCUS retrobulbar spot sign (RBSS; also known as the “intra-arterial spot sign”) can assist in differentiating this patient’s CRAO from other pathologies with similar presentations such as giant cell arteritis or retinal detachment.
The RBSS shows up as a bright, hyperechoic spot in the occluded artery immediately posterior to the globe. Think of it as analogous to the “hyperdense MCA” sign seen for stroke on CT. It is thought to represent calcium in the context of embolic causes of CRAO, and has been associated with poor response to thrombolysis and poor subsequent recovery of vision[14].
Published sensitivity and specificity of the RBSS approaches 83 and 100%, respectively.
Ensure you do not confuse the RBSS with benign optic disc drusen, which is reflective of calcium in the optic disc and occurs within the borders of the globe itself.
Take Home Point – In patients with acute painless vision loss, look for a retrobulbar spot sign as a specific sign indicative of embolic CRAO. Remember that absence of the RBSS does not rule out CRAO, and also not to misinterpret optic disc drusen as the RBSS.
Summary
POCUS can be a useful adjunct to the eye exam when seeing a patient with visual disturbances in the emergency department. It can be useful in detecting increased optic nerve sheath diameter and optic disc elevation suggestive of increased intracranial pressure. It may also be useful in seeing a thick or thin hyperechoic membrane which may suggest retinal detachment or vitreous detachment, respectively. In patients with vitreous hemorrhage, you may see a washing machine sign. Finally, look out for the retrobulbar spot sign indicative of central retinal artery occlusion. On your next shift, try using POCUS to help you gain more information to integrate into your clinical case and help you diagnose your ocular emergencies.
[1] Miller DL, Abo A, Abramowicz JS, Bigelow TA, Dalecki D, Dickman E, Donlon J, Harris G, Nomura J. Diagnostic Ultrasound Safety Review for Point-of-Care Ultrasound Practitioners. J Ultrasound Med. 2020 Jun;39(6):1069-1084. doi: 10.1002/jum.15202. Epub 2019 Dec 23. PMID: 31868252. [2] Michael Y. Woo, Nathan Hecht, Bernard Hurley, David Stitt, Venkatesh Thiruganasambandamoorthy, Test characteristics of point-of-care ultrasonography for the diagnosis of acute posterior ocular pathology, Canadian Journal of Ophthalmology, Volume 51, Issue 5, 2016, Pages 336-341, ISSN 0008-4182. https://doi.org/10.1016/j.jcjo.2016.03.020. (https://www.sciencedirect.com/science/article/pii/S0008418216303866) [3] Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med. 2003 Apr;10(4):376-81. doi: 10.1111/j.1553-2712.2003.tb01352.x. PMID: 12670853. [4] Kimberly HH, Shah S, Marill K, Noble V. Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 2008 Feb;15(2):201-4. doi: 10.1111/j.1553-2712.2007.00031.x. PMID: 18275454. [5] Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007 Apr;49(4):508-14. doi: 10.1016/j.annemergmed.2006.06.040. Epub 2006 Sep 25. PMID: 16997419. [6] Aletreby W, Alharthy A, Brindley PG, Kutsogiannis DJ, Faqihi F, Alzayer W, Balhahmar A, Soliman I, Hamido H, Alqahtani SA, Karakitsos D, Blaivas M. Optic Nerve Sheath Diameter Ultrasound for Raised Intracranial Pressure: A Literature Review and Meta-analysis of its Diagnostic Accuracy. J Ultrasound Med. 2022 Mar;41(3):585-595. doi: 10.1002/jum.15732. Epub 2021 Apr 24. PMID: 33893746. [7] Tessaro MO, Friedman N, Al-Sani F, Gauthey M, Maguire B, Davis A. Pediatric point-of-care ultrasound of optic disc elevation for increased intracranial pressure: A pilot study. Am J Emerg Med. 2021 Nov;49:18-23. doi: 10.1016/j.ajem.2021.05.051. Epub 2021 May 21. PMID: 34051397. [8] Teismann N, Lenaghan P, Nolan R, Stein J, Green A. Point-of-care ocular ultrasound to detect optic disc swelling. Acad Emerg Med. 2013 Sep;20(9):920-5. doi: 10.1111/acem.12206. PMID: 24050798. [9] Optic nerve sheath diameter. Saving Lives With Sound. (2021, July 15). Retrieved August 4, 2022, from https://www.albertasono.ca/neuro-ultrasound/optic-nerve-sheath-diameter/ [10] Josh FarkasJosh is the creator of PulmCrit.org. He is an associate professor of Pulmonary and Critical Care Medicine at the University of Vermont. (2017, October 28). Pulmcrit: Algorithm for diagnosing ICP elevation with ocular sonography. EMCrit Project. Retrieved August 4, 2022, from https://emcrit.org/pulmcrit/pulmcrit-algorithm-diagnosing-icp-elevation-ocular-sonography/ [11] Zoerle T, Caccioppola A, D’Angelo E, Carbonara M, Conte G, Avignone S, Zanier ER, Birg T, Ortolano F, Triulzi F, Stocchetti N. Optic Nerve Sheath Diameter is not Related to Intracranial Pressure in Subarachnoid Hemorrhage Patients. Neurocrit Care. 2020 Oct;33(2):491-498. doi: 10.1007/s12028-020-00970-y. PMID: 32314244. [12] Gottlieb M, Holladay D, Peksa GD. Point-of-Care Ocular Ultrasound for the Diagnosis of Retinal Detachment: A Systematic Review and Meta-Analysis. Acad Emerg Med. 2019 Aug;26(8):931-939. doi: 10.1111/acem.13682. Epub 2019 Feb 5. PMID: 30636351. [13 Lahham S, Shniter I, Thompson M, Le D, Chadha T, Mailhot T, Kang TL, Chiem A, Tseeng S, Fox JC. Point-of-Care Ultrasonography in the Diagnosis of Retinal Detachment, Vitreous Hemorrhage, and Vitreous Detachment in the Emergency Department. JAMA Netw Open. 2019 Apr 5;2(4):e192162. doi: 10.1001/jamanetworkopen.2019.2162. PMID: 30977855; PMCID: PMC6481597. [14] Altmann M, Ertl M, Helbig H, Schömig B, Bogdahn U, Gamulescu MA, Schlachetzki F. Low endogenous recanalization in embolic central retinal artery occlusion—The retrobulbar “spot sign”. Journal of Neuroimaging. 2015 Mar;25(2):251-6. [15] Cozzi N, Stevens K, Pillay Y, Moore D, Flannigan M, Barnes M, Singh M, Gagrica M, Kolacki C, Bach J, McNinch D. Diagnosis of Central Retinal Artery Occlusion in the Emergency Department Using POCUS: A Case Series. POCUS Journal. 2021 Nov 23;6(2):73-5. References







