How many times on shift do you see a patient that is on an anticoagulant or antiplatelet agent? The answer is probably almost always. The use of these agents and their complications are extremely common. And in particular cases, such intra-cerebral hemorrhage (~15-20%) (1), or unstable trauma patients, having an approach to reversal of these agents can be life-altering.
Two important questions for any bleeding patient on these agents:
1. When should I reverse?
2. What is the agent?
This post will review indications for reversal in the emergency room patient and provide you with an up-to-date, evidence-informed approach to anticoagulation (Warfarin, DOACs) and antiplatelet reversal for your next shift.
When should I consider reversal?
It is our role in the ED to understand how these drugs and their reversal agents in order to help patients, and you should consider reversal in all major or life-threatening bleeds.
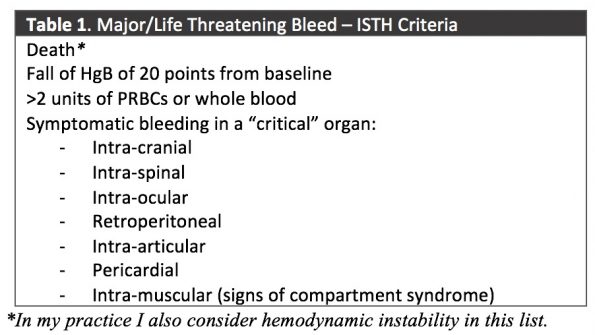
However, there are many definitions of what constitutes a major or life-threatening bleed. In my practice I use the criteria set by the International Society for Thrombosis and Haemostasis (ISTH), which is endorsed by Thrombosis Canada (2):
What is the agent?
Warfarin
Wafarin inhibits vitamin K epoxide reductase complex 1 in the liver, an enzyme that is used to activate vitamin K to be used as a co-factor in the body. By inhibiting this enzyme, there is a functional loss of vitamin K, leading to reduced production of vitamin-K dependent coagulation factors – X, IX, VII, II, protein C&S.
Since warfarin induces anticoagulation by inhibiting the synthesis of these factors, the simple and easy reversal strategy is to replace them.
One of the common uses of Warfarin is in patients with mechanical heart valves, which makes emergency physicians hesitate on reversal due to the potential risk of clotting events off these agents. However, evidence suggests that the immediate risks of thrombotic complications are largely overstated. In the short-term, i.e. within 2-weeks, there is no increased risk of thromboembolic events (3).
Therefore, all patients on Warfarin therapy with mechanical valves should be reversed. Involve your thrombosis colleagues early to help arrange re-starting therapy after the acute phase.
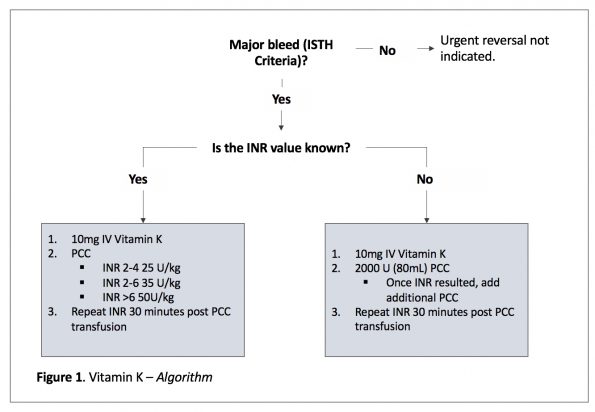
STEP 1: Vitamin K
All patients should receive 10mg IV of Vitamin K.
This overcomes the loss of vitamin K due to enzymatic inhibition. It has shown to be effective at reducing INR and preventing hematoma expansion in patients with major bleeding (4,5).
IV administration is important due to the peak effects at 4-6 hours (compared to PO at 24-36 hours) and IM/SC should be avoided due to erratic uptake (6).
STEP 2: Prothrombin Complex Concentrate
Based on the available evidence, PCC should be your first line agent in vitamin K antagonist bleeding, with ideal dosing based on your initial INR value.
Given the uptake time of IV Vitamin K (4-6 hours), and depletion of those associated factors (X, IX, VII, II, protein C&S), PCC should be given as an adjunct.
Three small randomized non-inferiority plasma control studies have been done looking at FFP vs 4-factor PCCs for reversal of warfarin (7,8,9). These studies demonstrate that PCC is faster at correcting INR (within 30-40 minutes) and is superior at achieving hemostasis, with less adverse events given it’s reduced volume of infusion. Both agents have a similar 2-week thrombosis rate (3-4%).
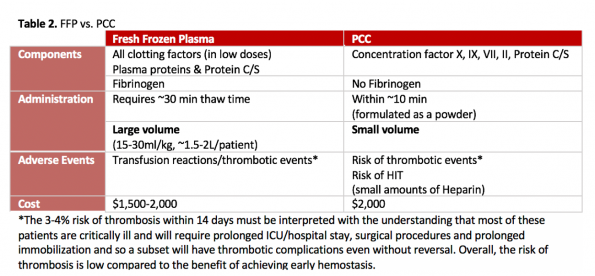
It is important to note that while there may be a trend towards mortality benefit with PCC, none of these studies are powered to detect this outcome. Additionally, there is no high-quality evidence comparing PCC to placebo focusing on patient-centered outcomes.
Intuitively though it does make sense that if we stop bleeding earlier by rapidly correcting the INR that we can stabilize clot formation and prevent worse outcomes. For example, Huttner et al (10) demonstrated rapid correction of INR within 2 hours is an independent predictor of improved survival, Tazaoroute et al (11) found a 2-fold increase in 7-day mortality in patients who did not receive Warfarin reversal, and a 2017 systematic review demonstrated a reduced all cause-mortality in patients given PCC over FFP (12).
Dosing of PCC
There is no universally agreed upon dosing strategy for 4-factor PCC for anticoagulation reversal, so this may vary depending on your institution. Below is the most commonly used strategy in the literature:
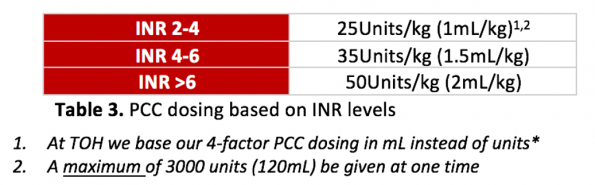
If the initial INR value is NOT KNOWN, then it is reasonable to give 2000 units of 4-factor PCCs upfront, and adjust after your INR value is resulted.
Your INR should be repeated in 30 minutes to assess for biochemical hemostasis.
Take Home Point: Warfarin reversal includes 10mg IV Vitamin K with 4-factor PCC, dosed based on your initial INR. If unknown, 2000U of PCC can be given up front.
Direct Oral Anticoagulants
There are two types of DOACs:
- Xa inhibitors (e.g., Apixaban, Rivaroxaban, Edoxaban) which inhibit factor Xa
- Direct Thrombin Inhibitors (e.g., Dabigatran) which inhibit factor II
Compared to warfarin, DOACs have a demonstrated clinical (reduced risk of stroke, ICH, overall mortality) and practical advantage (rapid onset, short-half life, ease of use/monitoring, etc.). One exception is Rivaroxaban and higher dose Dabigatran, which are associated with an increased risk of gastrointestinal bleeding.
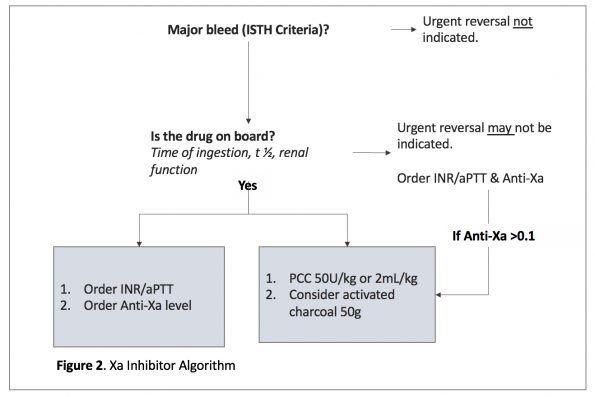
STEP 1: Does this patient have the drug in their system?
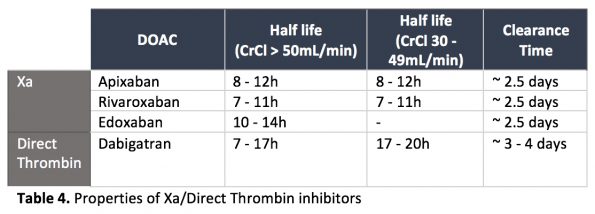
DOACs have a more reliable and shorter half-life than Warfarin. If we know when the drug was taken, the drug half-life, and the patient’s approximate renal function, we can calculate the time it would take to clear. In general, DOACs (apart from Dabigatran), do not accumulate in patients with CrCl above 30mL/min.
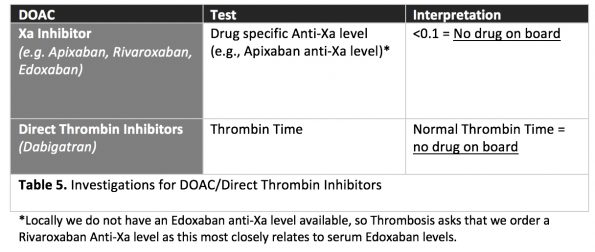
There are also specific tests available to order to determine if a patient has a clinically significant DOAC level in the blood:
STEP 2: Consider Activated Charcoal
Activated charcoal (AC) has been shown to reduce serum DOAC levels by up to 50% when given within 2 hours after DOAC administration; and up to 25% reduced if given 6 hours after DOAC administration. AC also shortens the elimination half-life of DOAC from 13.5h -> 5h (13).
All the usual contraindications for AC remain the same as they would for any toxicologic patient. However, it is a relatively safe and effective way to reduce the effect of DOACs.
Therefore, unless there are contraindications, AC should be given to patients presenting with major bleeding on a DOAC administered within 4-6 hours.
STEP 3: Anti-Xa vs. Direct Thrombin Inhibitor (Dabigatran)?
Anti-Xa
4-Factor PCC
4-factor PCC is the preferred reversal agent compared to FFP for Anti-Xa related reversal over FFP. It is not a true reversal agent, but works by overwhelming the coagulation cascade with exogenous factors to generate thrombin.
Dosing is 50Units/kg or 2mL/kg
Unlike warfarin, there is no upper limit for single dose of 4-factor PCC.
What’s the evidence?
Unfortunately, our evidence base is limited to a few non-randomized trials and have no control arms to compare 4-factor PCC to (14,15,16).
What we can take away is that PCC administration is effective at achieving hemostasis, with an overall risk of thrombotic events (2-4% within 14 days of administration). However, without a control arm it is difficult to say if the TEs are secondary to PCC or secondary to critical illness/natural course of their disease.
Other Targeted Therapies
Andexanet alfa is a recombinant factor Xa which acts as a ‘decoy receptor’ for Xa-inhibitors and low molecular weight heparins at a cost of $USD 25,000 – 50,000/dose (17).
Currently, Andexanet Alfa is not approved in Canada.
The best evidence we have supporting this medication comes from the 2018 ANNEXA-4 trial. This multi-centre prospective cohort study demonstrated a rapid correct of coagulation parameters with similar mortality rates as PCCs, yet a higher risk of TEs (10% at 30 days) (18).
However, there are several methodological flaws – no control arm, multiple protocol amendments, heavily industry sponsored, and a sensitivity analysis demonstrating no relationship between Xa and hemostasis (except for ICH patients). This makes interpretation of it’s effectiveness difficult.
Therefore, at this moment there is no clear evidence to use Andexanet Alfa over PCC for Xa-inhibitor reversal. Further trials compare PCC to Andexanet would be beneficial and are on-going.
Take Home Point: 4-factor PCC is the first line agent for Xa-inhibitor related major bleeding, in addition to your activated charcoal. Andexanet alfa is not available in Canada, and the evidence is limited (Figure 2):
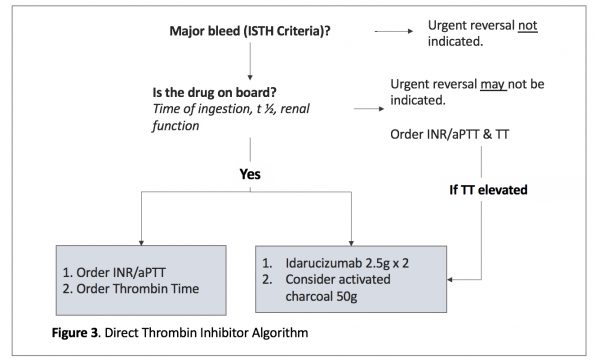
Direct Thrombin Inhibitors (Dabigatran)
Practically the only direct thrombin inhibitor encountered in the ED that will require reversal is dabigatran. Others do exist, however are often are administered via. infusion with extremely short half-lives.
While 4-factor PCC is an option for Dabigatran (Pradaxa) reversal, Dabigatran has a more specific and widely available reversal agent, Idarucizumab AKA Praxbind. Idarucizumab is a monoclonal antibody which binds serum dabigatran with 350X the affinity than dabigatran has for thrombin. It has a comparable cost to PCC (at TOH $2,250/5 grams vs. PCC at ~$2,000).
There are several properties that make it an ideal reversal agent:
- Rapid onset: works within seconds and has peak effect in minutes
- Short half-life: 45 mins
- Measurable effect: we can evaluate thrombin time before/during/after
- Littlle/no thrombotic event signal: due to its mechanism of binding to dabigatran, idarucizumab does not affect the coagulation cascade meaning there are limited side effects
- Prolonged effect: at 5g dosing, has been shown to have effect up to 72 hours administration (19,20,21)
Dosing is TWO separate 2.5 gram boluses (i.e., 5 GRAMS total). The blouses are run over 5-10 minutes with no more than 10-15 mins between boluses.
What’s the evidence?
The most important trial that resulted in its widespread use was the REVERSE-AD Trial, an international multi-centre prospective cohort study evaluating the reversal of dabigatran in patients with life threatening bleeding or requiring urgent surgery requiring normal hemostasis (22).
This 503-patient trial showed a 100% correction of direct thrombin time and cessation of bleeding within 2.5 hours with low mortality (13% at 30 days) and thrombotic rates (1% at 5 days, 5% at 30 days) after 5g of Idarucizumab. Despite its limitations – no control group, no specific definition of hemostasis, did not assess hemostasis for patients with ICH, lack of power to assess for important outcomes (i.e. mortality) – it demonstrates Idarucizumab is safe with little to no signal for TEs and clearly normalize coagulation parameters and achieves peri-procedural hemostasis (22).
Given similar cost, efficacy and better safety profile, Idarucizumab should be the first line choice for reversal in patients with major bleeding in the context of Dabigatran use over 4-factor PCC.
What if my centre does not have Praxbind?
If not available, activated charcoal and 4-factor PCC should be administered in the same fashion as for Xa inhibitors. Dabigatran is amenable to DIALYSIS. If there are delays in getting Idarucizumab, dialysis should be started.
Take Home Point: Dabigatran has a specific reversal agent, Idarucizumab (Praxbind). If not available, you can also use activated charcoal and 4-factor PCC, with consideration of dialysis.
Anti-platelet agents
Given the increase in interventional vascular procedures (e.g. PCI, EVT, vascular stents), so too is the frequency at which we encounter patients on dual anti-platelet therapy (DAPT).
DAPT carries a 2%/year risk of moderate-severe bleeding. Given this, there is a large degree of interest in reversal agents for anti-platelet therapy.
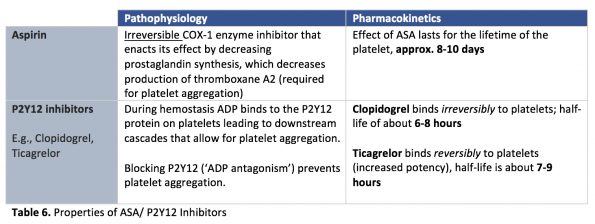
There are two types of anti-platelet agents, Aspirin & P2Y12 inhibitors:
What’s the current evidence for ED reversal?
DDAVP
Desmopressin acetate (DDAVP) is a vasopressin analog. In vivo it increases endothelial release of factor VIII and vWF. It also enhances platelet aggregation, which is the proposed mechanism behind its use as an anti-platelet agent reversal.
The Society of Critical Care Medicine and the European guidelines on bleeding and coagulopathy in trauma suggest DDAVP may be used for management of antithrombotic related bleeding. It is important to understand however, the evidence used to support DDAVP in these guidelines is mostly based on laboratory evidence (23,24).
Suggested dosing of DDAVP: 0.4ug/kg.
However, there is no high-quality evidence that administration of DDAVP improves bleeding time/platelet aggregation, and it should not be routinely given in the ED.
Two retrospective cohort studies in patients with ICH and TBI-related bleeding showed prevention of ICH and hematoma expansion, respectively, but no change in mortality, mRS or GCS status (25, 26). Alternatively, Schmidt et al (27) and McManus et al (28) demonstrated no difference in hematoma expansion with ICH. Finally, two randomized controlled trials in patients undergoing cardiac surgery did not show any benefit of DDAVP for reduction of blood loss (29, 30).
Platelet Transfusion
If a patient has dysfunctional platelets, why not just give them working platelets? This is the idea behind platelet transfusion for reversal of antiplatelet agents. For many decades, this was standard practice.
The pendulum has swung many times on this issue and most data comes from small studies of patients with traumatic or primary ICH. Some studies have found no difference in outcomes or hematoma expansion following platelet administration (31, 32), while others with significant methodological limitations have shown minimal benefit (33, 34).
One of the most conclusive and high-quality evidence we have against platelet transfusion comes from the PATCH trial by Baharoglu et al. This 190-patient multicentre open label randomized trial focused on patients with spontaneous ICH on anti-platelet therapy demonstrating a shift towards death or dependence on mRS at 3 months in the transfusion group (OR 2.05 95% CI 1.18 – 3.56, p=0.011). Reported adverse events and mortality were also higher in the transfusion arm.
Overall, there is good evidence that platelet transfusion for the sole purpose of anti-platelet reversal is associated with harm and should not be done. Platelet transfusion can still be considered in other disease states that cause platelet damage or dysfunction (e.g., DIC, TTP, uremia).
Take Home Point: DDAVP has minimal evidence in support of its use and should not be routinely given in the ED. Platelet transfusion is associated with harm and should not be given for the purposes of reversal.
Take Home Points
- Anticoagulation complications are common and ED physicians should know how to treat them.
- Apply ISTH criteria to any patient with bleeding on an anticoagulant to determine if urgent reversal is required.
- Standard warfarin reversal includes 10mg IV Vitamin K with INR based dosing of 4-factor PCC. If INR is unknown 2000U of PCC can be given up front.
- 4-factor PCC is the first line agent for Xa-inhibitor related major bleeding.
- Idarucizumab is the first line agent for dabigatran related major bleeding.
- Thrombotic event rates are low in patients reversed with PCC and likely an overstated risk in this critically ill population and should not delay time to administration of a reversal agent. The faster coagulation is normalized, likely the better the outcome for the patient!
- DDAVP is not recommended for anti-platelet reversal in the ED.
- Platelet transfusion for reversal of anti-platelet agents is associated with HARM and shouldn’t be considered as part of standard therapy.
- Reversal is only one part of achieving hemostasis. It is not the be all and end all. Reversal will not change the underlying pathology that cause bleeding in the first place, it will only allow for an environment that is more suitable to clot formation. We cannot forget about standard resuscitation measures and source control in patients with major bleeding in the ED.
References
- Béjot, Y., Cordonnier, C., Durier, J., Aboa-Eboulé, C., Rouaud, O., & Giroud, M. (2013). Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain, 136(2), 658-664.
- Schulman, S., Kearon, C., & Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. (2005). Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. Journal of thrombosis and haemostasis, 3(4), 692-694.
- Kuramatsu, J. B., Sembill, J. A., Gerner, S. T., Sprügel, M. I., Hagen, M., Roeder, S. S., … & Huttner, H. B. (2018). Management of therapeutic anticoagulation in patients with intracerebral haemorrhage and mechanical heart valves. European heart journal, 39(19), 1709-1723.
- Tsu, L. V., Dienes, J. E., & Dager, W. E. (2012). Vitamin K dosing to reverse warfarin based on INR, route of administration, and home warfarin dose in the acute/critical care setting. Annals of Pharmacotherapy, 46(12), 1617-1626.
- Raj, G., Kumar, R., & McKinney, W. P. (1999). Time course of reversal of anticoagulant effect of warfarin by intravenous and subcutaneous phytonadione. Archives of internal medicine, 159(22), 2721-2724.
- Holbrook, A., Schulman, S., Witt, D. M., Vandvik, P. O., Fish, J., Kovacs, M. J., … & Guyatt, G. H. (2012). Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest, 141(2), e152S-e184S.
- Sarode, R., Milling Jr, T. J., Refaai, M. A., Mangione, A., Schneider, A., Durn, B. L., & Goldstein, J. N. (2013). Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation, 128(11), 1234-1243.
- Goldstein, J. N., Refaai, M. A., Milling Jr, T. J., Lewis, B., Goldberg-Alberts, R., Hug, B. A., & Sarode, R. (2015). Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. The Lancet, 385(9982), 2077-2087.
- Steiner, T., Poli, S., Griebe, M., Hüsing, J., Hajda, J., Freiberger, A., … & Veltkamp, R. (2016). Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. The Lancet Neurology, 15(6), 566-573.
- Huttner, H. B., Schellinger, P. D., Hartmann, M., Köhrmann, M., Juettler, E., Wikner, J., … & Steiner, T. (2006). Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke, 37(6), 1465-1470.
- Tazarourte, K., Riou, B., Tremey, B., Samama, C. M., Vicaut, É., & Vigué, B. (2014). Guideline-concordant administration of prothrombin complex concentrate and vitamin K is associated with decreased mortality in patients with severe bleeding under vitamin K antagonist treatment (EPAHK study). Critical Care, 18(2), 1-9.
- Chai-Adisaksopha, C., Hillis, C., Siegal, D. M., Movilla, R., Heddle, N., Iorio, A., & Crowther, M. (2016). Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal A systematic review and meta-analysis. Thrombosis and haemostasis, 116(11), 879-890.
- Wang, X., Mondal, S., Wang, J., Tirucherai, G., Zhang, D., Boyd, R. A., & Frost, C. (2014). Effect of activated charcoal on apixaban pharmacokinetics in healthy subjects. American Journal of Cardiovascular Drugs, 14(2), 147-154.
- Majeed, A., Ågren, A., Holmström, M., Bruzelius, M., Chaireti, R., Odeberg, J., … & Schulman, S. (2017). Management of rivaroxaban-or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood, The Journal of the American Society of Hematology, 130(15), 1706-1712.
- Schulman, S., Gross, P. L., Ritchie, B., Nahirniak, S., Lin, Y., Lieberman, L., … & Study Investigators. (2018). Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thrombosis and haemostasis, 118(05), 842-851.
- Panos, N. G., Cook, A. M., John, S., & Jones, G. M. (2020). Factor Xa inhibitor-related intracranial hemorrhage: results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation, 141(21), 1681-1689.
- Micieli, A., Demchuk, A. M., & Wijeysundera, H. C. (2021). Economic evaluation of andexanet versus prothrombin Complex concentrate for reversal of factor Xa-associated intracranial hemorrhage. Stroke, 52(4), 1390-1397.
- Connolly, S. J., Crowther, M., Eikelboom, J. W., Gibson, C. M., Curnutte, J. T., Lawrence, J. H., … & Milling Jr, T. J. (2019). Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. New England Journal of Medicine, 380(14), 1326-1335.
- Glund, S., Stangier, J., Schmohl, M., Gansser, D., Norris, S., van Ryn, J., … & Kreuzer, J. (2015). Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial. The Lancet, 386(9994), 680-690.
- Glund, S., Stangier, J., Schmohl, M., Moschetti, V., Haazen, W., De Smet, M., … & Reilly, P. (2014). Idarucizumab, a specific antidote for dabigatran: immediate, complete and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Blood, 124(21), 344.
- Glund, S., Moschetti, V., Norris, S., Stangier, J., Schmohl, M., van Ryn, J., … & Reilly, P. (2015). A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thrombosis and haemostasis, 113(05), 943-951.
- Pollack Jr, C. V., Reilly, P. A., Van Ryn, J., Eikelboom, J. W., Glund, S., Bernstein, R. A., … & Weitz, J. I. (2017). Idarucizumab for dabigatran reversal—full cohort analysis. New England Journal of Medicine, 377(5), 431-441.
- Rossaint, R., Bouillon, B., Cerny, V., Coats, T. J., Duranteau, J., Fernández-Mondéjar, E., … & Spahn, D. R. (2016). The European guideline on management of major bleeding and coagulopathy following trauma. Critical care, 20(1), 1-55.
- Frontera, J. A., Lewin III, J. J., Rabinstein, A. A., Aisiku, I. P., Alexandrov, A. W., Cook, A. M., … & Zerfoss, C. L. (2016). Guideline for reversal of antithrombotics in intracranial hemorrhage. Neurocritical care, 24(1), 6-46.
- Feldman, E. A., Meola, G., Zyck, S., Miller, C. D., Krishnamurthy, S., Cwikla, G. M., … & Seabury, R. (2019). Retrospective assessment of desmopressin effectiveness and safety in patients with antiplatelet-associated intracranial hemorrhage. Critical care medicine, 47(12), 1759-1765.
- Barletta, J. F., Abdul-Rahman, D., Hall, S. T., Mangram, A. J., Dzandu, J. K., Frontera, J. A., & Zach, V. (2020). The role of desmopressin on hematoma expansion in patients with mild traumatic brain injury prescribed pre-injury antiplatelet medications. Neurocritical Care, 33(2), 405-413.
- Schmidt, K. J., Sager, B., Zachariah, J., Raad, B. F., James, E. G., & Fletcher, J. J. (2019). Cohort analysis of desmopressin effect on hematoma expansion in patients with spontaneous intracerebral hemorrhage and documented pre-ictus antiplatelet use. Journal of Clinical Neuroscience, 66, 33-37.
- McManus, J., Ferreira, J., Jones, G. M., Smetana, K. S., Condeni, M. S., Berger, K., … & Erdman, M. J. (2022). Effect of desmopressin acetate on acute spontaneous intracranial hemorrhage in patients on antiplatelet therapy. Journal of the Neurological Sciences, 434, 120142.
- Clagett, G. P., Valentine, R. J., Myers, S. I., Chervu, A., & Heller, J. (1995). Does desmopressin improve hemostasis and reduce blood loss from aortic surgery? A randomized, double-blind study. Journal of vascular surgery, 22(3), 223-230.
- Ansell, J., Klassen, V., Lew, R., Ball, S., Weinstein, M., VanderSalm, T., … & Salzman, P. (1992). Does desmopressin acetate prophylaxis reduce blood loss after valvular heart operations?: A randomized, double-blind study. The Journal of Thoracic and Cardiovascular Surgery, 104(1), 117-123.
- Downey, D. M., Monson, B., Butler, K. L., Fortuna Jr, G. R., Saxe, J. M., Dolan, J. P., … & McCarthy, M. C. (2009). Does platelet administration affect mortality in elderly head-injured patients taking antiplatelet medications?. The American surgeon, 75(11), 1100-1103.
- Ducruet, A. F., Hickman, Z. L., Zacharia, B. E., Grobelny, B. T., DeRosa, P. A., Landes, E., … & Connolly, E. S. (2010). Impact of platelet transfusion on hematoma expansion in patients receiving antiplatelet agents before intracerebral hemorrhage. Neurological research, 32(7), 706-710.
- Naidech, A. M., Liebling, S. M., Rosenberg, N. F., Lindholm, P. F., Bernstein, R. A., Batjer, H. H., … & Kwaan, H. C. (2012). Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocritical care, 16(1), 82-87.
- Li, X., Sun, Z., Zhao, W., Zhang, J., Chen, J., Li, Y., … & Zhang, W. (2013). Effect of acetylsalicylic acid usage and platelet transfusion on postoperative hemorrhage and activities of daily living in patients with acute intracerebral hemorrhage. Journal of neurosurgery, 118(1), 94-103.
- Baharoglu, M. I., Cordonnier, C., Salman, R. A. S., De Gans, K., Koopman, M. M., Brand, A., … & PATCH Investigators. (2016). Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. The Lancet, 387(10038), 2605-2613.