Physiologically difficult airway: airway management in the critically ill patient, whose underlying physiology puts them at higher risk of cardiovascular collapse with intubation and conversion to positive pressure ventilation.
This is important for in the Emergency Department as airway management is fundamental in the care of critically ill patients, but is not without risk – in fact it carries among the highest rate of life threatening complications of common critical care procedures:
- Risk of cardiac arrest following emergency intubation 1 in 25 (compared to 1 in 10 000 quoted in Anesthesia literature)
- Risk of severe cardiovascular collapse in patients who were hemodynamically stable prior to intubation — 30%
Predictors of cardiovascular collapse after endotracheal intubation:
- Hypoxemia
- Pre-intubation hypotension
- Shock index
This would suggest that a one-size-fits-all approach to intubation should not be the norm and that we should take patient factors into account when planning emergency intubations to avoid preventable critical outcomes.
Hypoxemia
- Most common complication of endotracheal intubation in the critically ill
- Desaturation < 70% puts patients at risk for:
- dysrhythmia
- hemodynamic decompensation
- hypoxic brain injury
- cardiac arrest and death
- Patients who are critically ill desaturate more rapidly due to pre-existing cardiopulmonary pathology, anemia, low cardiac output, VQ mismatch, and hypermetabolic states
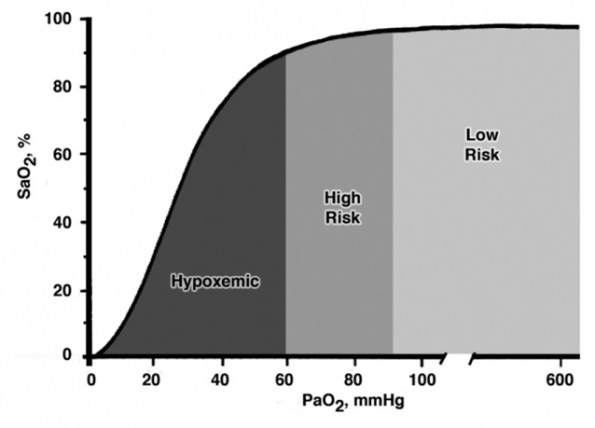
- Hypoxic patients have PaO2 values already on the steep portion of the pulse oximetry curve, and should be considered at high risk for desaturation
Preoxygenation
The next time you are managing a hypoxemic airway there are a few aspects to consider:
Prerequisite for endotracheal intubation, extends the duration of safe apnea. Several methods have been described:
Non-rebreather mask
- Only deliver 60-70% FiO2 at flow rates of 15 L/min; can be increased up to 90% with flow rates of 30-60L/min (beyond the calibrated markings of the oxygen source)
- FiO2 can also be increased with the addition of a 2nd source of O2 via nasal cannula at 15 L/min
- Alternatively, you can also use BVM with tight seal (2-handed technique), but this can be claustrophobic for awake patient
High-flow nasal cannula (HFNC)
- Provide FiO2 up to 100%, heated and humidified delivery of flow rates up to 60 L/min
- don’t entrain as much room air during inspiration and flush expired air from upper airway during respiration
- can generate PEEP (up to 5 cm H2O)
- Evidence for their use for pre-oxygenation has so far been mixed:
- Miguel-Montanes (2015) found significantly improved pre-oxygenation in patients with mild to moderate hypoxemia compared with NRB (2% vs 14%); patients were excluded if O2 sat < 95% on NRB prior to enrollment.
- Subsequent randomized trial (Vourc’h 2015) of hypoxemic ICU patients however concluded that HFNC as a pre-oxygenation device did not reduce the lowest level of desturation compared with NRB (91.5% vs 89.5%).
- Of note, HFNC was left in place during intubation attempts for apneic oxygenation in both of these studies.
Non-invasive ventilation
- If patients don’t achieve saturation > 93-95% after 3 min of pre-oxygenation, they are more likely to desaturate during apnea and intubation due to shunt physiology of the alveoli; this can be partially overcome by increasing mean airway pressure.
- A randomized study by Baillard (2005) found that NIV for pre-oxygenation was more effective at reducing desaturation during intubation than usual practice:
- patients pre-oxygenated with NIV had higher oxygen saturations before intubation (98% vs 93%) and were less likely to desaturate (<80%) during intubation.
- Positive pressure can also be applied with PEEP valve applied to the exhalation port of a standard bag mask with tight seal.
- This will only provide PEEP with expiration, however the addition of nasal cannula at 15 L/min as well will provide continuous PEEP.
Positioning
OR studies have shown that pre-oxygenation is improved and apnea time is prolonged in the back-up head elevated position, (see below) optimizing several aspects of the procedure:
- offloaded weight from the chest may improve ventilation and increase oxygen reserves
- improved views of the glottis with direct laryngoscopy have been described (Lee 2007)
- theoretical benefit of decreased risk of passive regurgitation in this position.
A study of 528 out-of-OR intubations (Khandelwal 2016) found a 13% absolute reduction in complications for patients intubated in this position. This benefit was preserved even when results were adjusted for BMI and predictors of difficult intubation.
These results are self-reported, subject to reporting bias and intubations using video laryngoscopy were excluded, which invites an element of selection bias if these devices were chosen for anticipated difficult airways. A prospective RCT is needed to confirm these findings, but this is an easy maneuver to do to optimize several elements of airway management, with no added cost or risk to your patients. Reverse Trendelenberg positioning may also be useful in patients who can’t bend at the waist or shoulders, as in those in whom here is concern for possible spinal injury.
Apneic oxygenation
In apneic patients, the alveolar gas deficit leads to net flow of gas from the pharynx into the alveoli. With a patent upper airway, increasing FiO2 externally provides a higher amount of O2 to the alveoli, with the intention of extending the duration of safe apnea time. This has been described in several OR studies of healthy patients undergoing elective intubation:
- An observational study by Frumin (1959) of intubated patients described no desaturation for up to 55 minutes (however association with very elevated CO2 levels and acidemia)
- A blinded crossover RCT by Teller (1988) found that during pharyngeal insufflation with 3L by nasal catheter, O2 sats never fell below 97% during a 10 minute apneic period.
- A 2010 study by Ramachandran randomized obese men undergoing elective intubation to receive apneic oxygenation at 5L/min or nothing and found the time to desaturation below 95% to be significantly prolonged (5.5 min vs 3.5 min), with a higher minimum O2 sat as well (94% vs 87%).
Increasing flow rates of nasal O2 administration up to 15L/min ensures delivery of set flow to pharynx. This was shown to be safe with no adverse events (Brainard 2015). The use of high-flow nasal cannula with heated humidified O2 also eliminates the concern for dessication and discomfort.
For emergent intubation, 2 major studies have examined apneic oxygenation:
A recently published observational study in the ED by Sakles (2016) found that ApOx was associated with increased odds of first-pass success for intubation without hypoxemia (82% vs 69%). The authors’ conclusion is that ApOx prolongs safe apnea time, preventing multiple intubation attempts that have been shown to be associated with increased risk of adverse events. This study is limited to a single-centre ED and is subject to reporting bias as data was collected from forms completed by the intubator following the procedure.
The only RCT to date evaluating ApOx for emergent intubations was done by Semler (2016). ICU patients were randomized to nasal cannula 15 L/min vs usual care (no ApOx). They found no difference in median lowest arterial saturation (92% vs 90%) or incidence of desaturation <90% (45% vs 47%). Thorough review of the data included in the supplement reveals that the majority of patients were not left apneic prior to intubation – 40% of patients received BVM ventilation after induction medications were pushed and 1/3 of patients had BiPAP (not true RSI). There was also no standardized method to ensure airways was patent during ApOx
In summary, there appears to be no harm to apneic oxygenation, but there may not be benefit in patients requiring NIV or BVM ventilation for preoxygenation, given the shunt physiology exhibited.
Hypoxemia – Summary
- Pre-oxygenation and apneic oxygenation should be performed in all critically ill patients
- O2 saturation 96-100%: NRB with max flow rate or BVM + nasal cannula at 15 L/min OR high-flow nasal cannula
- O2 saturation < 95%: CPAP or BVM with PEEP to improve alveolar recruitment and oxygenation
- Position all patients in back-up head elevated position
- Apneic oxygenation is a low-risk intervention with no proven harms that may provide significant benefit in prolonging the safe apneic period, however they may be no benefit in patients requiring NIV or BVM for preoxygenation
Hemodynamic instability
– Several physiologic reasons for hypotension after emergent endotracheal intubation: decreased preload given decreased venous return from the initiation of positive pressure ventilation, attenuation of catecholamine surge with resolution of hypoxia/hypercarbia (especially in hypovolemia), vasodilation and myocardial depression due to induction agents, and underlying pathology (cardiopulmonary, acid-base, sepsis, hemorrhage, hypovolemia).
– A retrospective study of intubations in a large urban ED (Heffner 2012) found the incidence of postintubation hypotension in patients who were hemodynamically stable before intubation to be 23% (almost 1 in 4 patients). This was independently associated with a higher risk of in-hospital mortality and longer ICU stay. Immediate pre-RSI shock index was the variable most strongly associated with post-intubation hypotension (OR 55). Other factors included ESRD and age.
– A Canadian study out of Halifax by Green et al. identified that 44% of patients intubated in the ED developed post-intubation hemodynamic instability. Increasing age, COPD and pre-intubation hemodynamic instability were identified as associated risk factors.
Shock index
- Presence of hypotension before intubation predicts catastrophic consequences in the postintubation period; however, arterial pressure is well regulated by reflex compensatory mechanisms and so does not begin to change until late in the progression toward cardiovascular collapse.
- An elevated shock index (SI = HR/sBP; normal < 0.7) is an early sign of shock despite otherwise normal vitals. A shock index >0.9 has been shown to correlate with severity of illness, associated with mortality and need for therapeutic intervention
- In the context of emergent intubation, 2 studies have examined SI. Besides the Heffner study, a retrospective analysis of 140 normotensive patients intubated emergently in the ICU (Trivedi 2015) found a significant association between a preintubation SI >0.9 and the development of sBP less than 90 mmHg in postintubation period. Patients with elevated SI also had higher mortality rate. Further studies are needed to evaluate the role of SI as a variable in prognostic scoring systems, but this is a quick and noninvasive way of predicting who is at risk for deterioration in the peri-intubation period and in whom you should intervene before intubation.
Resuscitate before you intubate
- Establish at least 2 proximal peripheral IVs; can give RSI medications intraosseusly if necessary, as demonstrated in prospective study from a combat hospital in Afghanistan (Barnard 2015), which showed no difference in first-pass success or laryngoscopic view with meds administered via IO.
- Volume loading (at least 500mL if patient isn’t in pulmonary edema; 1L wide open recommended) +/- blood in hemorrhagic shock. Increase in circulating volume will increase mean systemic pressure and venous return; if right heart can accommodate increased venous return, cardiac output will increase.
RV failure
- In RV failure, afterload is increased du to chronic pulmonary hypertension from lung or left heart disease, or pulmonary arterial hypertension. Chronically, this leads to increased contractility and preload. If the RV is unable to meet increased demands acutely, there is dilatation, retrograde flow and decreased coronary perfusion, hypotension and cardiovascular collapse
- Intubation in these patients is extremely risky: changes in intrathoracic pressure have exaggerated effect on hemodynamics and RV function worsens with positive pressure ventilation (PEEP increases RV afterload further and can compress pulmonary vasculature). This can lead to cardiovascular collapse
- RV afterload is decreased by ensuring good preoxygenation and apneic oxygenation prior to intubation
- Point of care ultrasound can be used to identify patients who might be at risk
- In RV failure, there is a risk of volume overload with even a small fluid challenge: the pressure overloaded RV can increase diastolic wall tension and LV diastolic dysfunction, which leads to decreased LV filling and stroke volume. Cardiac output is impaired due to ventricular interdependence , when the septum is pushed into LV, leading to impaired function. It is better to resuscitate with vasopressors – norepinephrine is the pressor most extensively studied, could also use epinephrine; important to avoid phenylephrine as it will worsen pulmonary vasoconstriction, further increasing RV afterload.
Peri-intubation pressor support
- If your patient is hypotensive – start norepinephrine before you intubate, anticipating hemodynamics will worsen.
- If not hypotensive, consider bolus dose phenylephrine – pure vasoconstrictor (for vascular tone until medications wear off). A retrospective study (Panchal 2015) of 119 hypotensive patients requiring intubation who had received push-dose phenylephrine peripherally pre- or peri-intubation found a 20% increase in BP with no effect on heart rate.
- Bolus dose epinephrine is also an option as it will also provide improved cardiac output.
- Peripherally infused pressors can be administered safely in the ED: a recent review article by Loubani identified risk factors for extravasation – IV site distal to antecubital/popliteal fossa and prolonged infusion (average 60 hours).
Medications
- Use of topical lidocaine spray may be all that is needed in severe hemodynamic compromise.
- All induction agents can contribute to hypotension due to decreased vascular tone and reduced venous return; propofol and benzodiazepines will also decrease sympathetic tone, leading to myocardial depression. Hemodynamically stable agents are recommended, such as etomidate and ketamine. A comparison of the 2 for intubation in sepsis found no difference in serious complications.
- While the literature is sparse, a review article of ketamine (Morris 2009) concluded that it represents a “very rational choice for RSI in hemodynamically compromised patients”, including those with brain injury. Ketamine acts as sympathomimetic in patients with intact autonomic nervous system. Important to use with caution though – while it is less likely to cause hypotension than propofol, a pre-hospital study (Miller 2016) of 112 patients found that ketamine was associated with hypotension in patients with shock index >0.9 prior to induction. This reinforces the importance of resuscitation prior to intubation. Patients who developed hypotension likely had depleted catecholamine stores and limited sympathetic reserve.
- Dosage adjustment: recommend at least 50% reduction in induction dose for at-risk patients in uncompensated shock.
- The use of neuromuscular blocking agents has been independently associated with reduced risk of post-intubation hypotension. Paralytic agents will take longer to work in shock state as they are dependent on cardiac output and need to get to peripheral musculature – higher doses may be required.
Hypotension – Summary
- Resuscitate before you intubate: calculate the shock index (HR/sBP) to identify patients at risk for post-intubation hypotension and cardiovascular collapse; use caution in right ventricular failure.
- Thoughtful choice of hemodynamically stable RSI medications.
- Early vasopressor support
Severe metabolic acidosis
In the case of acidemia due to severe metabolic acidosis (DKA, ASA toxicity, severe lactic acidosis), homeostasis depends on respiratory compensation from alveolar hyperventilation. When organic acid production demands ventilation requirement that can’t be met, profound acidemia can result. In these patients, even a brief apneic period can lead to a profound drop in pH given loss of respiratory compensation
- A retrospective case series from the NYC poison centre of 7 patients who received mechanical ventilation in the setting of ASA poisoning found that all patients reviewed had marked decrease in pH and increase in PCO2 after institution of mechanical ventilation, leading to death in 2 patients (Stolbach 2008).
- Mechanical ventilator sometimes can’t match pre-intubation alveolar ventilation – would recommend avoiding intubation if possible – short trial of NIV may help to support work of breathing until correction of metabolic acidosis can occur.
Bicarbonate therapy
- Proponents of bicarbonate argue that a low pH is harmful (most notably due to impaired cardiovascular function), and if bicarb can increase the pH when infused IV, why wouldn’t you give it?
- A review published in Chest (Forsythe 2000) examined the literature for the use of sodium bicarbonate for the treatment of lactic acidosis. There is no evidence that venous or arterial pH is representative of cellular pH. The mounting evidence in patients ventilated with permissive hypercapnia in lung-protective ventilation strategies doesn’t correlate with depressed cardiac output in acidemia, in fact these patients tolerate the acidemia well.
- Furthermore, while it has been shown that IV bicarbonate can raise the blood pH, it is not clear whether this leads to an increase in cellular pH. CO2 produced in the blood as bicarbonate reacts with metabolic acids can diffuse readily across cell membranes while bicarbonate does not, which could in fact decrease intracellular pH further. No controlled studies have shown improved hemodynamics or catecholamine responsiveness due to sodium bicarbonate infusion.
- This is obviously not the case in salicylate poisoning, where sodium bicarb infusion in the mainstay of treatment for alkalinization of plasma and urine.
Pearls for airway management
- Maintaining spontaneous respiration is key, so that the patient can maintain their own high minute ventilation – consider awake intubation in these patients.
- AVOID RSI – if you must, should choose succinylcholine (short-acting neuromuscular blocker) and low-dose sedatives – minimize time that the patient’s ventilatory drive is compromised.
- Measure end-tidal CO2 prior to induction and target this number, along with their pre-intubation RR on the vent.
- Frequent blood gas monitoring
- Ventilator-assisted preoxygenation – trial out of Australia combines nasal oxygen at 15 Lpm and NIV for preoxygenation and pressure controlled ventilation during apnea (Grant 2016). Ventilation during onset of muscular relaxation can avoid or delay the rate of increase of PaCO2 during apnea and avoid cardiovascular collapse.
- When setting up the ventilator, consider pressure-controlled ventilation modes that allow the patient to set the rate and tidal volume received to maintain their respiratory compensation; monitor for air trapping
Metabolic acidosis – Summary
- Avoid intubation if possible
- IV bicarbonate probably won’t help as patient is already breathing at their maximum
- Apnea kills, consider awake intubation or ventilator-assisted preoxygenation and induction
Summary
- Recognition of the physiologically difficult airway is key to safe airway management, and taking the time to optimize conditions before intubation will save your patient’s life
- For the hypoxic patient: preoxygenate, either with NRB, HFNC or NIV depending on severity of hypoxia, so that they don’t fall off that oxyhemoglobin cliff. Apply nasal cannula at 15 Lpm for apneic oxygenation to prolong safe apnea time. Start in head-up back elevated position for improved views and possibly decreased complication rate.
- For the hemodynamically unstable patient: recognize those at risk using the shock index; establish access, give fluids (be wary of RV failure), start pressors before you intubate if hypotensive or at least have push-dose pressors ready in anticipation, use hemodynamically stable induction medications at reduced dose and high-dose paralytics
- For the patient with severe metabolic acidosis: apnea kills – avoid intubation if at all possible. If it must be done, consider awake fiberoptic intubation and call for help if you need it
References
Baillard C et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. Am J Respir Crit Care Med. 2006. 174:171-177
Barnard EBG et al. Rapid sequence induction of anaesthesia via the intraosseus route: a prospective observational study. Emerg Med J. 2015. 32:449-452
Brainard A et al. A randomized trial on subject tolerance and the adverse effects associated with higher vs lower-flow oxygen through a standard nasal cannula. Annals of Emergency Medicine. 2015. 65(4):356-361
Forsythe SM & Schmidt GA. Sodium bicarbonate for the treatment of lactic acidosis. Chest. 2000. 117:260-267
Frumin MJ et al. Apneic oxygenation in man. Anesthesiology. 1959. 20:789-798
Futier E et al. Noninvasive ventilation and alveolar recruitment maneuver improve respiratory function during and after intubation of morbidly obese patients. Anesthesiology. 2011. 114(6):1354-1363
Grant S et al. Ventilator-assisted pre-oxygenation: protocol for combining non-invasive ventilation and apnoeic oxygenation using a portable ventilator. Emergency Medicine Australasia. 2016. 28:67-72
Green RS et al. Evaluation of the incidence, risk factors and impact on patient outcomes of postintubation hemodynamic instability. CJEM. 2012. 14(2): 74-82
Heffner AC et al. Incidence and factors associated with cardiac arrest complicating emergency airway management. Resuscitation. 2013. 84:1500-1504
Heffner AC et al. Predictors of the complication of postintubation hypotension during emergency airway management. Journal of Critical Care. 2012. 27:587-593
Heffner AC et al. The frequency and significance of postintubation hypotension during emergency airway management. Journal of Critical Care. 2012. 27:417.e9-417.e13
Jaber S et al. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-centre study. Intensive Care Med. 2010. 36:248-255
Khandelwal N et al. Head-elevated patient positioning decreases complications of emergent tracheal intubation in the ward and intensive care unit. Anesth Analg. 2016. 122:1101-1107
Kim WY et al. Factors associated with the occurrence of cardiac arrest after emergency tracheal intubation in the emergency department. PLoS One. 2014. 9(11):e112779
Krishnan S & Schmidt GA. Acute right ventricular dysfunction. Chest. 2015. 147(3):835-846
Lee BJ et al. Laryngeal exposure during laryngoscopy is better in the 25 degree back-up position than in the supine position. British Journal of Anaesthesia. 2007. 99(4): 581-586
Loubani OM & Green RS. A systematic review of extravasation and local tissue injury from administration of vasopressors through peripheral intravenous catheters and central venous catheters. Journal of Critical Care. 2015. 30(3):653.e9-653.e17
Miguel-Montanes R et al. Use of high-flow nasal cannula oxygen therapy to prevent desaturation during tracheal intubation of intensive care patients with mild-to-moderate hypoxemia. Crit Care Med J. 2015. 43(3):574-583
Miller M et al. Hemodynamic response after rapid sequence induction with ketamine in out-of-hospital patients at risk of shock as defined by the shock index. Annals of Emergency Medicine. 2016. 68(2):181-188
Morris C et al. Anaesthesia in hemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009. 64:532-539
Mort TC. Preoxygenation in critically ill patients requiring emergency tracheal intubation. Crit Care Med. 2005. 33(11): 2672-2675
Mosier JM et al. The physiologically difficult airway. Western Journal of Emergency Medicine. 2015. 16(7):1109-1117
Panchal AR et al. Efficacy of bolus-dose phenylephrine for peri-intubation hypotension. The Journal of Emergency Medicine. 2015. 49(4): 488-494
Perbet S et al. Incidence of and risk factors for severe cardiovascular collapse after endotracheal intubation in the ICU: a multicenter observational study. Critical Care. 2015. 19:257
Ramachandran SK et al. Apneic oxygenation during prolonged laryngoscopy in obese patients: a randomized controlled trial of nasal oxygen administration. Journal of Clinical Anesthesia. 2010. 22:164-168
Sakles JC et al. First pass success without hypoxemia is increased with the use of apneic oxygenation during rapid sequence intubation in the emergency department. Academic Emergency Medicine. 2016. 23:703-710
Semler MW et al. Randomized trial of apneic oxygenation during endotracheal intubation of the critically ill. Am J Respir Crit Care Med. 2016. 193(3):273-280
Spoletini G et al. Heated humidified high-flow nasal oxygen in adults. Chest. 2015. 148(1):253-261
Stolbach AI et al. Mechanical ventilation was associated with acidemia in a case series of salicylate-poisoned patients. Academic Emergency Medicine. 2008. 15:866-869
Teller LE et al. Pharyngeal Insufflation of oxygen prevents arterial desaturation during apnea. Anesthesiology. 1988. 69:980-982
Trivedi S et al. Evaluation of preintubation shock index and modified shock index as predictors of postintubation hypotension and other short-term outcomes. Journal of Critical Care. 2015. 30:861.e1-861.e7
Vourc’h M et al. High-flow nasal cannula oxygen during endotracheal intubation in hypoxemic patients: a randomized controlled clinical trial. Intensive Care Med. 2015. 41:1538-1548
Weingart SD & Levitan RM. Preoxygenation and prevention of desaturation during emergency airway management. Annals of Emergency Medicine. 2012. 59(3): 165-175
Weingart SD. Push-Dose Pressors. http://emcrit.org/podcasts/bolus-dose-pressors/ Accessed January 19, 2017
Weingart SD. Vent as bag & VAPOX. http://emcrit.org/podcasts/emcrit-wee-vapox/ Accessed January 10, 2017





Trackbacks/Pingbacks