The Surviving Sepsis Guidelines define sepsis as a “life-threatening organ dysfunction caused by a dysregulated host response to an infection”. Septic shock is further defined as sepsis with hypotension (MAP ≤65) and/or lactate ≥2.0 despite adequate fluid resuscitation(1,2).
This definition of septic shock highlights the importance of factoring in both macrocirculatory (blood pressure) and microcirculatory markers (lactate) when treating patients. Current resuscitation targets include a MAP≥65mmHg and normalization of lactate (1,2).
Patients in sepsis are much more complicated than just those two numbers. If our ultimate goal is to improve tissue perfusion, many other markers should be utilized in the care of these patients including:
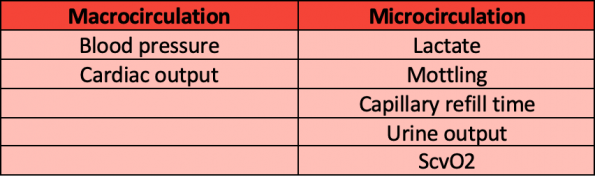
Macrocirculation in Septic Shock
Blood Pressure
Blood pressure (BP) has traditionally been used as a tool to guide our resuscitation. However, the goal of maintaining a MAP of 65mmHg is largely based on observational trials. There are some limitations to utilizing an optimal BP as a target for sepsis:
- BP targets may differ in those with chronic hypertension or other comorbidities.
- Targeting a BP may result in the unnecessary use of invasive BP monitoring via arterial lines and/or vasopressors.
- The use of vasopressors may reduce blood flow to our small vessels and consequently affect tissue perfusion.
Currently, there is still debate on optimal BP targets.
- SEPSISPAM was one of the first randomized controlled trials that compared higher BP targets (MAP 80 – 85) vs. lower BP targets (65 – 70mmHg). The results showed no difference in 28 or 90 day mortality in patients targeted to a higher BP(3).
- The 65 trial by Lamontagne et al randomized patients in septic shock to permissive hypotension (via reduction of vasopressor use). This study showed no difference in mortality in patients in the hypotensive group (MAP 60 – 65) vs. traditional therapy(4)
These two studies highlight that BP is an imperfect measure of perfusion.
Cardiac Output
Cardiac output is another common macrocirculatory measurement. However, its utility in the ED is limited by a lack of objective numbers. Point of care ultrasound (POCUS) has helped significantly in this regard and can be used as a supplement to assess for gross cardiac output.
Microcirculation in Septic Shock
The use of microcirculation markers in guiding resuscitation in septic shock has increased in the past decade(5–9). There is evidence that decreased small vessel perfusion leads to unfavourable outcomes:
- De Backer et. al showed that decreased perfusion of small vessels is significantly associated with mortality in patients with septic shock(5).
- Hernandez et al. showed that mortality increased in the lowest quartile of perfusion(7).
- These studies both showed, however, that there was no significant relationship between MAP and mortality, as well as MAP and small vessel perfusion.
This highlights the importance of focusing on small vessel perfusion since microcirculation is likely the primary driving force in tissue perfusion.
Lactate
The Surviving Sepsis Campaign (2018) recommended measuring lactate in it’s “Hour-1 Bundle of Care” and repeating it in 2 – 4hrs if it is ≥2mmol/L(1). The question we must ask ourselves when interpreting an elevated lactate is whether this is due to a Type A (inadequate oxygen delivery) or Type B (no evidence of inadequate oxygen delivery) process(10).
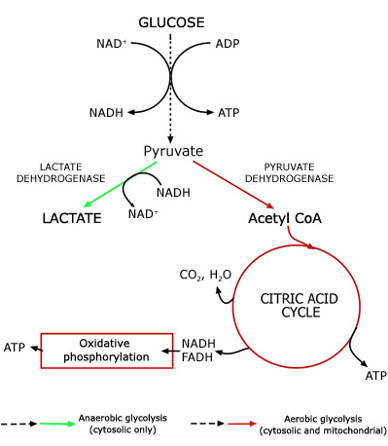
The metabolism of lactate is complex and not completely understood:
- Glycolysis begins → pyruvate is formed
- Pyruvate is then broken down to lactate via lactate dehydrogenase
- This releases NAD+ to allow for further glycolysis
Thus, lactate generation is a “necessary evil” to allow glycolysis to continue.
Lactate use for risk stratification in septic shock
Lactate has been shown to help risk-stratify patients with sepsis(11–18):
- Shapiro et. al showed an increased mortality in patients admitted with sepsis and a lactate >2.5. For patients with a lactate >4, they had an OR of 4.9 for mortality in 28 days, and an OR of 9.5 for mortality in 3 days. However, this study did not report data on hemodynamics, co-morbidities, or medication use, and should be interpreted as hypothesis generating(16).
- Mikkelson et al. studied the relationship between lactate (low <2, intermediate 2 – 3.9, high ≥4) and in-hospital mortality for patients with sepsis and septic shock(12). In the non-shock group, patients with intermediate and high lactates had an odds ratio of mortality rates at 28 days of 2 and 2.91 respectively. In the shock group, the odds ratio for were 4.41 and 5.14 for intermediate and high lactates respectively. Of note, this study had few patients with liver and renal dysfunction (~10%).
- Surviving Sepsis Guidelines supports the notion that a lactate ≥2 secondary to tissue hypoxia/hypoperfusion is associated with increased mortality in patients with sepsis.
Lactate clearance in septic shock
Surviving Sepsis Campaign (2016) suggests normalizing of lactate as a guidance to resuscitation. This lead to many other studies assessing lactate clearance and kinetics:
- Nguyen et al.reported a two-fold increase in mortality for patients who cleared <10% of their lactate in the first 6hrs – concluding an 11% decrease in mortality for every 10% of lactate clearance within the first 6hrs(19).
- Puskarich et al. showed that at 6hrs, both lactate normalization (OR 6.3, 95% CI 2.4 – 17.0) and lactate clearance of 50% (OR 4.3, 95% CI 1.8 – 10.2) were most associated with survival(20).
Overall, there is a growing body of evidence supporting the target of a normalization of lactate at the 6-hour mark. Surviving sepsis guidelines recommend repeating lactates every 2 – 4 hours. The literature supports repeating lactate measurements q2h targeting a 10 – 20% reduction with the goal of lactate normalization at 6hrs(1).
Some limitations to this guide to resuscitation include:
- Transfer of care of patients from the ED to the ICU or medicine unit
- Patients with impaired lactate clearance ability secondary to renal failure and liver failure.
Mottling and Capillary Refill Time
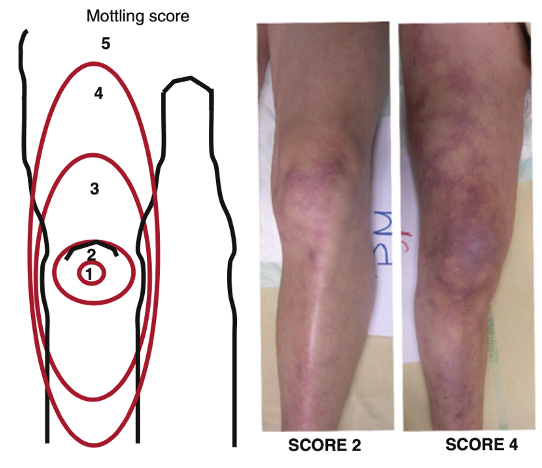
Mottling and capillary refill time (CRT) are two tools that can be used to risk stratify and guide resuscitation. Mottling is patchy skin discolouration that reflects blood flow reduction and tissue hypoperfusion – most easily assessed at the knees.
One way to assess mottling is by using the mottling score, developed by Dr. Ait-Oufella(21–23). An increase in the mottling score has been associated with an increased mortality (24,25). Important limitations to consider include the external temperature of patients and other causes of livedo reticularis. Patients with dark skin were excluded from the mottling score studies as they were more difficult to assess. As such, other markers of skin hypoperfusion should be used for these patients.
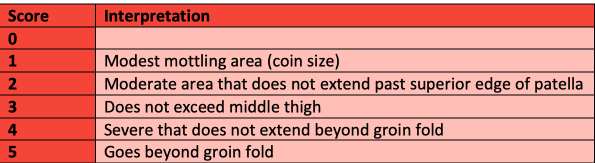
Capillary refill time (CRT) is another tool that has been used to assess tissue hypoperfusion(26–31). Assess CRT by applying pressure to the distal phalanx until it blanches, hold it for 10 seconds then use a stopwatch to record time the time it takes for colour to return.
ANDROMEDA- SHOCK trial
Numerous observational trials have looked at CRT guided resuscitation, which led to ANDRO-MEDA shock, the first multicenter RCT that randomized septic shock patients to lactate guided resuscitation vs. CRT guided resuscitation(28).

In this study, patients had their resuscitation targeted to either a normal CRT (≤3 seconds) or improvement of lactate (lactate ≤2mmol or reduction by 20% q2h). They utilized a step-by-step guide to accomplish this as shown below:
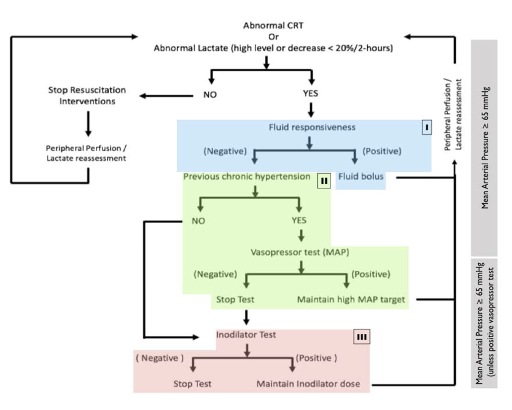
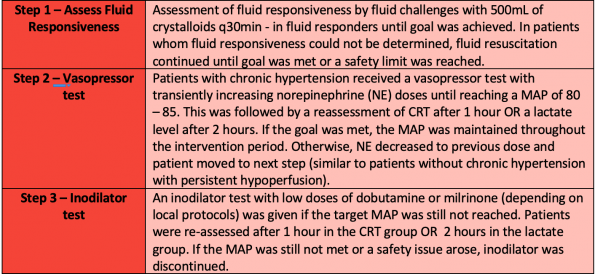
The results showed a trend towards improved 28d mortality in the CRT vs. lactate group (34.9% vs. 43.4%, p = 0.06), but failed to reach statistical significance. This was interpreted by some as CRT being non-inferior to lactate guide resuscitation. Shortly afterward, a Bayseian re-analysis of the trial was done.
Bayseian analysis of ANDROMEDA-SHOCK trial
What is a bayesian analysis? The posterior belief is a combination of the likelihood of an event being true and prior beliefs (“Bayes factor”). This is similar to what we do in clinical medicine. For example, you see a patient with chest pain. You take a focused history and perform a physical examination; from there you decide what the likelihood of this patient having an ischemic event is. You then perform tests such as an ECG, troponin, and a chest x-ray. You combine all that information together to form your “posterior belief” or your “post-test probability”.
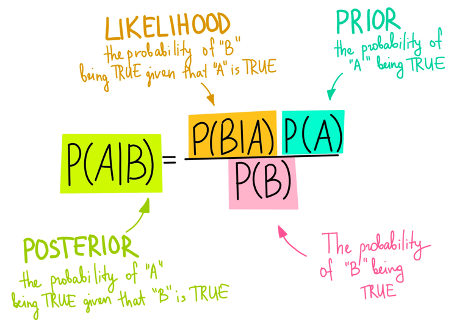
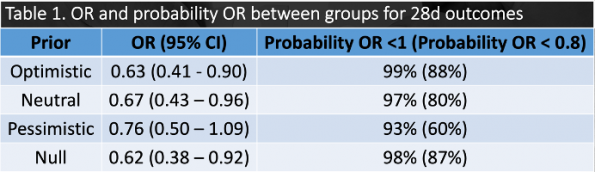
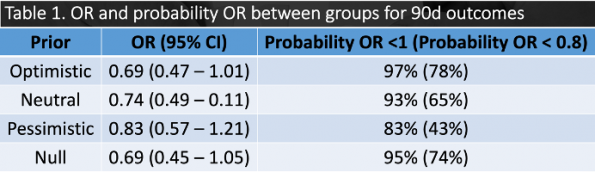
CRT vs. lactate guided resuscitation using four different priors was utilized (optimistic, pessimistic, null, neutral)(32). Results showed that regardless of which prior was used, the posterior probability favoured CRT in both 28d and 90d outcomes. When analyzing the original trial, one of the differences between the two groups was the amount of IVF received in the first 8hrs (CRT 2359mL vs. lactate 2767mL, p = 0.01). As such, CRT may prevent over-resuscitation of these patients with IV fluids, which has negative effects such as pulmonary edema.
Take Home Points
There is no perfect marker for perfusion in sepsis. These patients are complicated to manage, and there are many factors that you have to piece together to improve their perfusion. Some key points to remember include:
- MAP ≠ perfusion
-
- Ensure that you’re utilizing other markers to assess perfusion in conjunction with MAP
- Lactate ≥2 is a prognosticator of mortality in sepsis
-
- Consider differential for Type A vs. Type B lactic acidosis
- Lactate clearance is associated with improved survival
-
- Recommend repeating lactates q2h and targeting 10 – 20% clearance
- Goal is normalization at 6hrs
- Caution in patients with liver and renal failure
- Mottling score is associated with mortality
- CRT can be used in conjunction with lactate to guide resuscitation
References
- Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med. 2018;46(6):997–1000.
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Vol. 45, Critical Care Medicine. 2017. 486–552 p.
- Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, et al. High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014;370(17):1583–93.
- Lamontagne F, Richards-Belle A, Thomas K, Harrison DA, Sadique MZ, Grieve RD, et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients with Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA – J Am Med Assoc. 2020;
- De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: Impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41(3):791–9.
- Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(SUPPL. 4):13–9.
- Hernandez G, Boerma EC, Dubin A, Bruhn A, Koopmans M, Edul VK, et al. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care [Internet]. 2013;28(4):538.e9-538.e14. Available from: http://dx.doi.org/10.1016/j.jcrc.2012.11.022
- Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am J Physiol – Hear Circ Physiol. 2002;282(1 51-1):156–64.
- Prognostic-implications-of-multicirculatory-dysfunction.pdf.
- Hernandez G, Bellomo R, Bakker J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med [Internet]. 2019;45(1):82–5. Available from: https://doi.org/10.1007/s00134-018-5213-x
- Oedorf K, Day DE, Lior Y, Novack V, Sanchez LD, Wolfe RE, et al. Serum lactate predicts adverse outcomes in emergency department patients with and without infection. West J Emerg Med. 2017;18(2):258–66.
- Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah C V., et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37(5):1670–7.
- Bou Chebl R, El Khuri C, Shami A, Rajha E, Faris N, Bachir R, et al. Serum lactate is an independent predictor of hospital mortality in critically ill patients in the emergency department: A retrospective study. Scand J Trauma Resusc Emerg Med. 2017;25(1):1–7.
- Lee SW, Hong YS, Park DW, Choi SH, Moon SW, Park JS, et al. Lactic acidosis not hyperlactatemia as a predictor of inhospital mortality in septic emergency patients. Emerg Med J. 2008;25(10):659–65.
- Gotmaker R, Peake SL, Forbes A, Bellomo R. Mortality is Greater in Septic Patients with Hyperlactatemia Than with Refractory Hypotension. Shock. 2017;48(3):294–300.
- Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, et al. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45(5):524–8.
- Garcia-alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. 2014;1–11.
- Thomas-Rueddel DO, Poidinger B, Weiss M, Bach F, Dey K, Häberle H, et al. Hyperlactatemia is an independent predictor of mortality and denotes distinct subtypes of severe sepsis and septic shock. J Crit Care [Internet]. 2015;30(2):439.e1-439.e6. Available from: http://dx.doi.org/10.1016/j.jcrc.2014.10.027
- Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, et al. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(8):1637–42.
- Puskarich MA, Trzeciak S, Shapiro NI, Albers AB, Heffner AC, Kline JA, et al. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest [Internet]. 2013;143(6):1548–53. Available from: http://dx.doi.org/10.1378/chest.12-0878
- Galbois A, Bigé N, Pichereau C, Boëlle PY, Baudel JL, Bourcier S, et al. Exploration of skin perfusion in cirrhotic patients with septic shock. J Hepatol [Internet]. 2015;62(3):549–55. Available from: http://dx.doi.org/10.1016/j.jhep.2014.10.012
- Ait-Oufella H, Bourcier S, Alves M, Galbois A, Baudel JL, Margetis D, et al. Alteration of skin perfusion in mottling area during septic shock. Ann Intensive Care [Internet]. 2013;3(1):1–6. Available from: Annals of Intensive Care
- Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, et al. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37(5):801–7.
- Jouffroy R, Saade A, Tourtier JP, Gueye P, Bloch-Laine E, Ecollan P, et al. Skin mottling score and capillary refill time to assess mortality of septic shock since pre-hospital setting. Am J Emerg Med [Internet]. 2019;37(4):664–71. Available from: https://doi.org/10.1016/j.ajem.2018.07.010
- Dumas G, Lavillegrand JR, Joffre J, Bigé N, De-Moura EB, Baudel JL, et al. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit Care. 2019;23(1):1–9.
- Ait-Oufella H, Bige N, Boelle PY, Pichereau C, Alves M, Bertinchamp R, et al. Capillary refill time exploration during septic shock. Intensive Care Med. 2014;40(7):958–64.
- Hariri G, Joffre J, Leblanc G, Bonsey M, Lavillegrand JR, Urbina T, et al. Narrative review: clinical assessment of peripheral tissue perfusion in septic shock. Ann Intensive Care [Internet]. 2019;9(1):1–9. Available from: https://doi.org/10.1186/s13613-019-0511-1
- Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality among Patients with Septic Shock: The ANDROMEDA-SHOCK Randomized Clinical Trial. JAMA – J Am Med Assoc. 2019;321(7):654–64.
- Sheridan DC, Baker SD, Kayser SA, Jones D, Hansen ML. Variability of Capillary Refill Time among Physician Measurements. J Emerg Med. 2017;53(5):e51–7.
- Hernandez G, Pedreros C, Veas E, Bruhn A, Romero C, Rovegno M, et al. Evolution of peripheral vs metabolic perfusion parameters during septic shock resuscitation. A clinical-physiologic study. J Crit Care [Internet]. 2012;27(3):283–8. Available from: http://dx.doi.org/10.1016/j.jcrc.2011.05.024
- Lima A, Jansen TC, Van Bommel J, Ince C, Bakker J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. 2009;37(3):934–8.
- Zampieri FG, Damiani LP, Bakker J, et al. Effects of a Resuscitation Strategy Targeting Peripheral Perfusion Status versus Serum Lactate Levels among Patients with Septic Shock. A Bayesian Reanalysis of the ANDROMEDA-SHOCK Trial. Am J Respir Crit Care Med. 2020;201(4):423–429. doi:10.1164/rccm.201905-0968OC