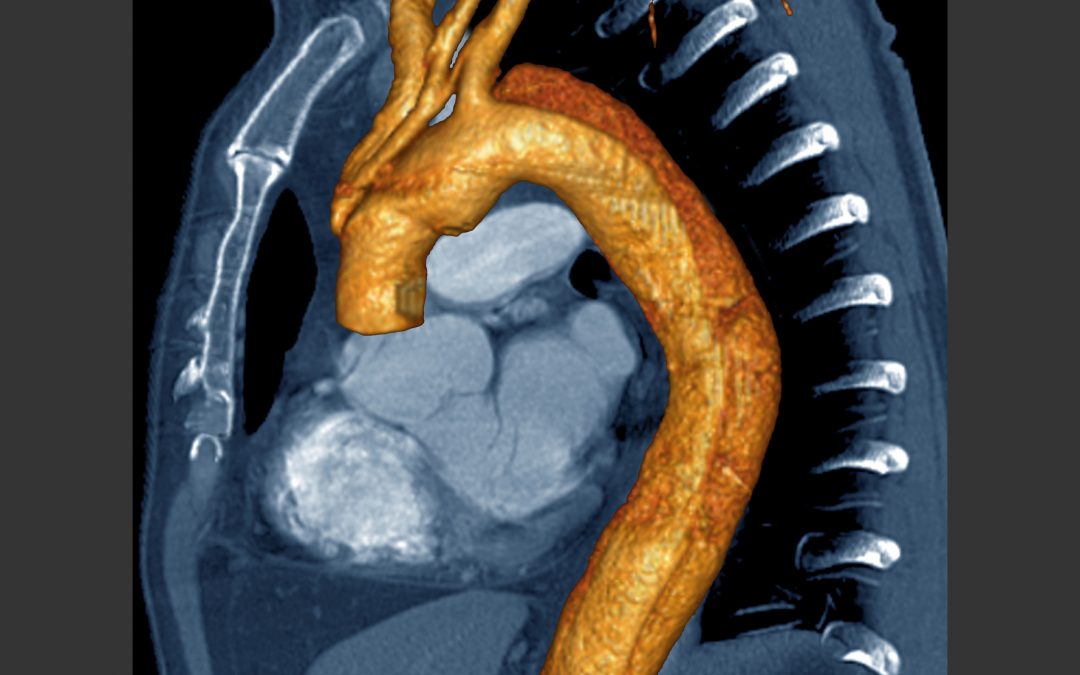
A 65-year-old male self-presents to the Emergency Department (ED) with sudden-onset severe chest pain. The pain was sharp, lasted 30 minutes, and has now resolved. His vitals and ECG are normal, and his high-sensitivity troponin testing is negative. He has a normal CXR, normal cardiac exam, and no pulse or neurologic deficits. You wonder … could this be an aortic dissection?
Staff Note
Post reviewed, agree with below.
Acute aortic syndrome is a HALO diagnosis (high acuity low occurrence). We cannot order a CT for everyone we see “just in case” and yet, we cannot discount the diagnosis simply because it is not common.
Dr. Wells provides an excellent summary of the evidence surrounding AAS. The single most important thing you can do as a physician to reduce your chance of missing this diagnosis is to ask and document all relevant high-risk pain features, risk factors, and physical exam findings. Other data points, such as D-dimer, point of care ultrasound, a chest x-ray can be potentially useful; however, without a complete history, they are only a small part of a larger picture. The biggest impact on your pre-test probability comes from what your patient is telling you.
– Dr. Robert Ohle
Acute Aortic Syndromes
Aortic dissection makes up one of the Acute Aortic Syndromes (AAS). The 3 diagnoses are considered as part of the same spectrum of disease and are investigated and treated similarly.3 4
- Aortic dissection ( 85-90% of AAS) – involves a tear of the intimal layer of the aorta, with the formation of a false lumen and anterograde or retrograde expansion
- Intramural hematoma (5-10% of AAS) – caused by a rupture of the vasa vasorum in the media, resulting in a thrombus within the aortic wall, which may extend or resolve spontaneously
- Penetrating atherosclerotic ulcer (~5%) – can lead to intramural thrombus, dissection, or aortic perforation
Aortic Dissection
Epidemiology
- Aortic dissection patients are approximately 2/3 male, with an average age of ~63.5
- Women typically present slightly later in life, with an average age of ~67.6
- Patients under 40 years of age make up about 5-10% of all dissections, and about 50% of these patients will have Marfan’s Syndrome.5
Classification
Most are familiar with the Stanford and DeBakey classification systems. The Stanford classification is more widely used, while the DeBakey system has more surgical relevance.
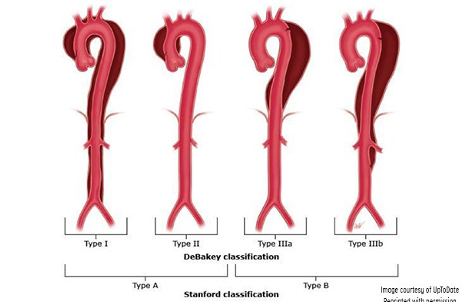
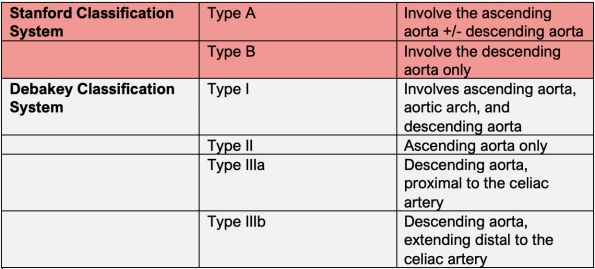
Type A dissections make up about 62% of all dissections. Most type A dissections extend to involve the descending aorta.5 9 Type A dissections have a higher in-hospital mortality rate (20-30%) than type B dissections (~5-10%) and are almost always treated surgically. Type B dissections carry significantly higher longer-term survival rates and can often be managed medically.10
Aortic dissection symptoms are due to:
- Dynamic obstruction of the true lumen by the false lumen, or,
- Extension of the false lumen to a vessel that branches off the aorta.1
Due to the large number of vessels that can be affected, the clinical presentation varies widely which makes it challenging to diagnose the condition. Rates of misdiagnosis for aortic dissection range from 14-38%11,12,13 A recent survey study by Ohle et al. of Canadian EP’s showed that ~2/3 of respondents would accept a <1% miss rate for aortic dissection.14
History
The first step for generating diagnostic suspicion of aortic dissection is to get a good, complete pain history.
1. Pain characteristics
- Chest/back pain (85-95%) with abrupt onset (85%) and severe intensity (90%).
- No pain (5-15%)
- Likely to present with neurologic symptoms, syncope, CHF, or tamponade instead.
- A lack of sudden onset chest pain ( -LHR of 0.3)19
- Migratory pain (+LHR 1.1-7.6)
- Tearing/ripping pain (+LHR 1.2-10.8), present in about 50%.9,19
- Sharp pain (~65%)
2. Risk factors
- Thoracic aortic aneurysm or abdominal aortic aneurysm
- Marfan’s syndrome
- Bicuspid aortic valve
- Family history of AD
- Recent aortic surgery or catheterization
A small study (N=83) by Rosman et al. found that asking about quality, location, and onset of pain increased the accuracy of initial clinical suspicion for AD.15When all 3 were asked, the diagnosis was suspected in 91%. When less than 3 questions were asked, dissection was suspected in only 49%. Another study by Ohle et al. found that a focused pain history led to fewer missed cases .16
Physical Examination
Neurologic symptoms
-
- New neurologic deficits + chest/back pain is the most concerning physical exam finding
- Present in 17-40% of type A dissections 20
- 5-10% of dissections present as presumed strokes21,22
- Highest +LHR for a physical exam finding, ranging from 6.6-3319
- Up to 50% of neurologic symptoms will be transient and may have resolved at ED presentation21
Peripheral pulse deficit
-
- Present in 15-31% of patients 23
- +LHR ranging from 2.4-5.719
- Pulse deficits need to be obtained from proximal pulses (carotid, brachial, femoral) to be considered true deficits
- Distal pulses are not reliable due to the incidence of peripheral vascular disease
Blood pressure (BP) differential
-
- BP differential >20mmHg between arms is historically reported as a significant clinical predictor
- Studies have demonstrated that 50% of patients without AD will have a BP differential >10mmHg between arms and 19% have a BP differential of >20 mmHg24
- Therefore, BP differential is less useful than a palpable pulse deficit23
Aortic regurgitation murmur
-
- Present in 30-50% of Type A dissections
- New murmurs it has a strong +LHR of 519
Blood pressure
-
- Type A dissection: hypotension, normotension, and hypertension present with similar frequencies9,25
- Type B dissections: most present hypertensive (~70%)10,21
ECG findings
-
- Non-specific ST segment or T wave changes (40%)5
- Ischemic changes and STEMI (15% of type A dissections)
- STEMI’s secondary to AD most commonly involve the RCA, but can involve any vessel
Diagnostic testing
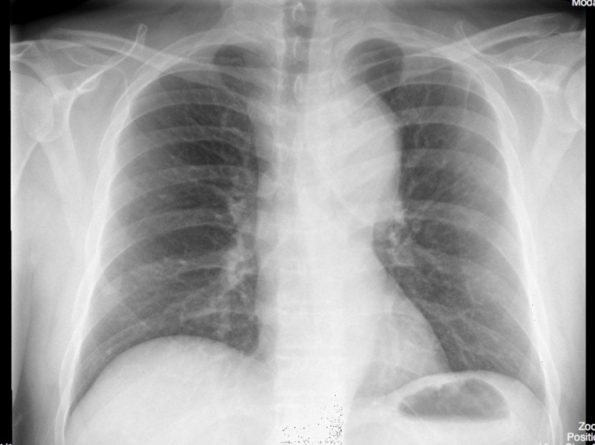
- Chest x-ray (CXR)
-
- A wide mediastinum is the most common finding seen in 60-90% of cases and associated with a +LHR 1.1-3.422
- It is measured at the level of the aortic knob: defined as ≥8 cm or >1/3rd of the transthoracic distance26
- Most CXRs in patients with AD will have abnormalities, only 10-20% are completely normal19
- A normal CXR has a negative LHR of 0.14-0.6.
- A wide mediastinum is the most common finding seen in 60-90% of cases and associated with a +LHR 1.1-3.422
Other signs of AD on CXR: ,27
-
- Calcium Sign – seen in 5-15%, defined as the separation of the intimal calcification from the outer aortic soft tissue (>10mm considered positive)28
- Abnormal/widened aortic contour
- Loss of aortic knob
- Loss of aortopulmonary (AP) window
- Left apical cap
- Left pleural effusion
- Loss of paratracheal stripe
- Rightwards tracheal deviation
- Downwards displacement of L mainstem bronchus
- Displacement of NG tube
2. Transthoracic echocardiography (TTE)
- TTE can be done using point-of-care ultrasound (POCUS)
- Suprasternal notch view and the apical 5-chamber view in addition to traditional cardiac views.
- POCUS should be the initial imaging modality in suspected AD, especially in unstable patients3
- TTE performance in trained hands allows detection of AD, but performs better for Type A dissections29
- Data from an old study from the 1980’s showed fairly good performance (indicated below), with newer studies reporting higher sensitivities and specificities 30
- Ascending aorta:
- Sensitivity: 78–90%
- Specificity: 87–96%
- Descending aorta:
- Sensitivity: 33-51%
- Specificity: 60-83%
- Ascending aorta:
- The finding that is essentially diagnostic for AD on TTE is the identification of an intimal flap29
- Discuss this finding with cardiac surgery promptly, as they may be able to forego CT imaging and go directly to the OR, with a pre-op transesophageal echo (TEE).
- This strategy is endorsed in the ACEP and European Society of Cardiology guidelines.3 32
- Other supportive findings include aortic regurgitation, pericardial effusion, and a dilated aortic root.
- Dilated aortic root >4cm (with clinical correlation) → highly suspicious for AD
- Patients presenting with pain and either aortic root dilatation or new aortic regurgitation on POCUS should be sent promptly for CTA.
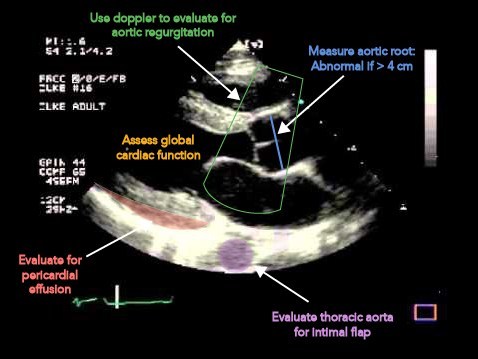
The optimal use of POCUS in the work-up of potential AD is as an initial imaging study, especially in unstable patients with suspected type A dissection. The modality should be used for its high specificity, allowing emergency physicians (EP) to rule in the condition early in the course of evaluation. POCUS should not be used to rule out AD.
3. Advanced diagnostic Imaging
- CT angiography, MRI aortography, or TEE all are highly sensitive and specific for the detection of AD.33,34
- Helical CT angiography has over 99% sensitivity and 98% specificity, however significant variation in CT ordering rates exists among EP’s and 95-98% of CT’s ordered to assess for AD are negative.33 35
- Dissection protocol CT scans have significant radiation loads and incidental findings.36 Therefore, the real issue lies in which patients with unexplained chest pain require CT imaging to assess for aortic dissection.
Risk scores and biomarkers
- Aortic Dissection Detection Risk Score (ADD-RS)
Biomarkers and risk stratification tools have been studied to rule out AD. In 2010, the American Heart Association developed the Aortic Dissection Detection Risk Score (ADD-RS) – the only decision rule to date that has been validated.37 Scores range from 0-3 points with a given point for the presence of 1 or more features in a single column.
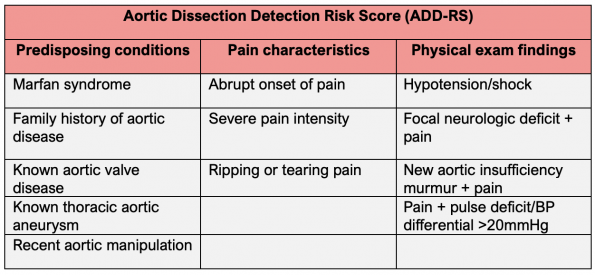
Scores of 0-1 are usually considered low risk, while scores of 2+ are considered high risk.
Significant issues with the ADD-RS
- Multiple components of this score should lead to CT if seen in isolation
- Patients with multiple findings in a single category still only get a score of 1, but their risk is likely much higher.
- Migratory pain and known abdominal aortic aneurysm were not included as a high-risk pain feature.
Rogers and colleagues applied the score to patients from the International Registry of Aortic Diseases (IRAD).38 They found a 95.7% sensitivity for scores of 1. Another study by Nazerian et al. found a sensitivity of 91.1% for scores of 1 when applying the score to patients suspected to have AD.39
2. D-Dimer
Asha et al. found that D-dimer alone has a pooled sensitivity of 98% for AD and a negative LHR of 0.05 upon a systematic review.40They proposed that this biomarker may be useful to rule out AD in a group of low-risk patients but D-dimer is limited by its low specificity. Available guidelines do not agree upon its current utility and there is a lack of perspective validation of its use in decision making and impact on CT usage rates.
ADVISED trial – A multicenter prospective observational study looking at combining D-Dimer with the ADD-RS for ruling out dissection.41 It used an ADD-RS ≤1 with a negative D-Dimer for ruling out AD. They included 1850 patients who were being worked up for possible AD. The prevalence of AD was 13%.
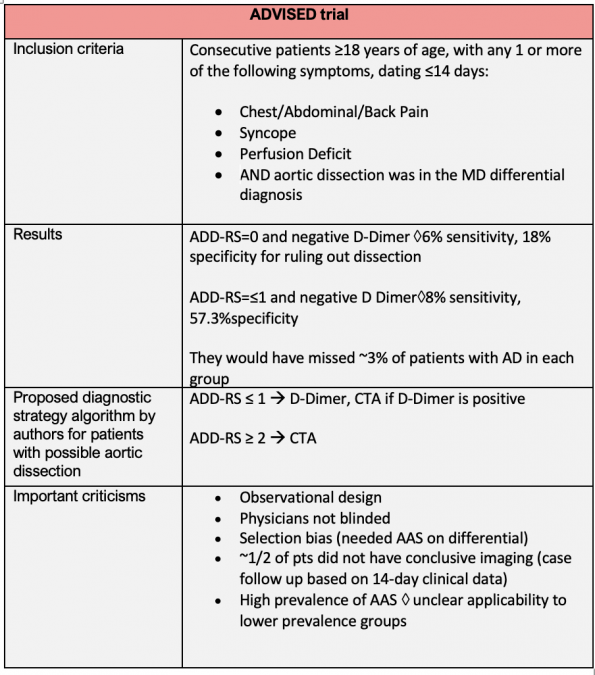
Additionally, there are a number of significant criticisms of this rule-out strategy. For example, arguably if you are considering AD in your differential, you likely have at least one high-risk feature. Most physicians would argue a patient with an ADD-RS of 0 has NO high-risk features, and therefore extremely low pretest probability. Therefore, ordering a D-Dimer for these patients is likely unnecessary and will lead to additional imaging studies with minimal change in the rate of disease detection.
Take home points
- ADD-RS or D-Dimer alone cannot rule-out AD
- Significant issues exist with the ADD-RS
- D-Dimer high a sensitivity, but a low specificity
- Utilizing a D-Dimer and ADD-RS risk score strategy for low risk patients will likely result in excessive CT imaging with low returns
- Low ADD-RS and negative D-Dimer show some potential in improving diagnostic accuracy, but this strategy is still not ready for primetime
- Unfortunately, patients presenting with no high-risk features will always be a diagnostic challenge
- At present, we should be relying on generating a good pain history, looking for high risk patient characteristics and physical exam findings, and using CXR or POCUS to help determine who needs a CTA to assess for AD.
Future directions
Be on the lookout for the new Canadian clinical practice guidelines on acute aortic syndromes, being developed by Dr. Robert Ohle and colleagues. They are soon to be published and may change the paradigm of how we work-up/rule out AD in the Emergency Department.
References
- Mészáros I, Mórocz J, Szlávi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117(5):1271-1278. doi:10.1378/chest.117.5.1271
- Clouse WD, Hallett JW, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176-180. doi:10.4065/79.2.176
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseasesDocument covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adultThe Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873-2926. doi:10.1093/eurheartj/ehu281
- Evangelista A, Czerny M, Nienaber C, et al. Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating aortic ulcer. Eur J Cardiothorac Surg. 2015;47(2):209-217. doi:10.1093/ejcts/ezu386
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): New Insights Into an Old Disease. JAMA. 2000;283(7):897-903. doi:10.1001/jama.283.7.897
- Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation. 2004;109(24):3014-3021. doi:10.1161/01.CIR.0000130644.78677.2C
- Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10(3):237-247. doi:10.1016/s0003-4975(10)65594-4
- DeBakey ME, Beall AC, Cooley DA, et al. Dissecting aneurysms of the aorta. Surg Clin North Am. 1966;46(4):1045-1055. doi:10.1016/s0039-6109(16)37946-4
- Collins J. Stewart, Evangelista Arturo, Nienaber Christoph A., et al. Differences in Clinical Presentation, Management, and Outcomes of Acute Type A Aortic Dissection in Patients With and Without Previous Cardiac Surgery. Circulation. 2004;110(11_suppl_1):II-237. doi:10.1161/01.CIR.0000138219.67028.2a
- Suzuki Toru, Mehta Rajendra H., Ince Hüseyin, et al. Clinical Profiles and Outcomes of Acute Type B Aortic Dissection in the Current Era: Lessons From the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108(10_suppl_1):II-312. doi:10.1161/01.cir.0000087386.07204.09
- Chua M, Ibrahim I, Neo X, Sorokin V, Shen L, Ooi SBS. Acute aortic dissection in the ED: risk factors and predictors for missed diagnosis. Am J Emerg Med. 2012;30(8):1622-1626. doi:10.1016/j.ajem.2011.11.017
- Asouhidou I, Asteri T. Acute aortic dissection: be aware of misdiagnosis. BMC Res Notes. 2009;2:25. doi:10.1186/1756-0500-2-25
- Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol. 2007;99(6):852-856. doi:10.1016/j.amjcard.2006.10.055
- Ohle R, McIsaac S, Yan J, Yadav K, Eagles D, Perry JJ. National survey of emergency physicians on the risk stratification and acceptable miss rate of acute aortic syndrome. CJEM. 2020;22(3):309-312. doi:10.1017/cem.2019.489
- Rosman HS, Patel S, Borzak S, Paone G, Retter K. Quality of History Taking in Patients With Aortic Dissection. Chest. 1998;114(3):793-795. doi:10.1378/chest.114.3.793
- Ohle R, Isaac SM, Perry JJ. A simple intervention to reduce your chance of missing an acute aortic dissection. Can J Emerg Med. 2019;21(5):618-621. doi:10.1017/cem.2019.1
- Januzzi JL, Eagle KA, Cooper JV, et al. Acute Aortic Dissection Presenting With Congestive Heart Failure: Results From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol. 2005;46(4):733-735. doi:10.1016/j.jacc.2005.05.023
- Helman A. Aortic Dissection | EM Cases | EM Cases Course. Emergency Medicine Cases. Published February 21, 2017. Accessed May 31, 2020. https://emergencymedicinecases.com/aortic-dissection-em-cases-course/
- Klompas M. Does This Patient Have an Acute Thoracic Aortic Dissection? JAMA. 2002;287(17):2262-2272. doi:10.1001/jama.287.17.2262
- Conzelmann LO, Hoffmann I, Blettner M, et al. Analysis of risk factors for neurological dysfunction in patients with acute aortic dissection type A: data from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg. 2012;42(3):557-565. doi:10.1093/ejcts/ezs025
- Gaul C, Dietrich W, Erbguth FJ. Neurological Symptoms in Aortic Dissection: A Challenge for Neurologists. Cerebrovasc Dis. 2008;26(1):1-8. doi:10.1159/000135646
- Ohle R, Kareemi HK, Wells G, Perry JJ. Clinical Examination for Acute Aortic Dissection: A Systematic Review and Meta-analysis. Acad Emerg Med. 2018;25(4):397-412. doi:10.1111/acem.13360
- Um SW, Ohle R, Perry JJ. Bilateral blood pressure differential as a clinical marker for acute aortic dissection in the emergency department. Emerg Med J. 2018;35(9):556-558. doi:10.1136/emermed-2018-207499
- Singer AJ, Hollander JE. Blood pressure. Assessment of interarm differences. Arch Intern Med. 1996;156(17):2005-2008. doi:10.1001/archinte.156.17.2005
- Golledge J, Eagle KA. Acute aortic dissection. The Lancet. 2008;372(9632):55-66. doi:10.1016/S0140-6736(08)60994-0
- Fultz PJ, Melville D, Ekanej A, et al. Nontraumatic rupture of the thoracic aorta: chest radiographic features of an often unrecognized condition. AJR Am J Roentgenol. 1998;171(2):351-357. doi:10.2214/ajr.171.2.9694450
- Earnest F, Muhm JR, Sheedy PF. Roentgenographic findings in thoracic aortic dissection. Mayo Clin Proc. 1979;54(1):43-50.
- Jagannath AS, Sos TA, Lockhart SH, Saddekni S, Sniderman KW. Aortic dissection: a statistical analysis of the usefulness of plain chest radiographic findings. AJR Am J Roentgenol. 1986;147(6):1123-1126. doi:10.2214/ajr.147.6.1123
- Evangelista A, Maldonado G, Gruosso D, et al. The current role of echocardiography in acute aortic syndrome. Echo Res Pract. 2019;6(2):R53-R63. doi:10.1530/ERP-18-0058
- Erbel R, Engberding R, Daniel W, Roelandt J, Visser C, Rennollet H. Echocardiography in diagnosis of aortic dissection. Lancet Lond Engl. 1989;1(8636):457-461. doi:10.1016/s0140-6736(89)91364-0
- Zarama V, Arango-Granados MC, Bustamante Cristancho LA. Importance of Multiple-window Assessment for the Diagnosis of Ascending Aortic Dissection Using Point-of-care Ultrasound: Report of Three Cases. Clin Pract Cases Emerg Med. 2019;3(4):333-337. doi:10.5811/cpcem.2019.6.43245
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Thoracic Aortic Dissection, Diercks DB, Promes SB, et al. Clinical policy: critical issues in the evaluation and management of adult patients with suspected acute nontraumatic thoracic aortic dissection. Ann Emerg Med. 2015;65(1):32-42.e12. doi:10.1016/j.annemergmed.2014.11.002
- Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic Accuracy of Transesophageal Echocardiography, Helical Computed Tomography, and Magnetic Resonance Imaging for Suspected Thoracic Aortic Dissection: Systematic Review and Meta-analysis. Arch Intern Med. 2006;166(13):1350-1356. doi:10.1001/archinte.166.13.1350
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1-9. doi:10.1056/NEJM199301073280101
- Ohle R, Anjum O, Bleeker H, Wells G, Perry JJ. Variation in emergency department use of computed tomography for investigation of acute aortic dissection. Emerg Radiol. 2018;25(3):293-298. doi:10.1007/s10140-018-1587-x
- Prabhakar AM, Le TQ, Abujudeh HH, Raja AS. Incidental findings and recommendations are common on ED CT angiography to evaluate for aortic dissection. Am J Emerg Med. 2015;33(11):1639-1641. doi:10.1016/j.ajem.2015.07.078
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA Guideline for Assessment of Cardiovascular Risk in Asymptomatic Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56(25):e50-e103. doi:10.1016/j.jacc.2010.09.001
- Rogers Adam M., Hermann Luke K., Booher Anna M., et al. Sensitivity of the Aortic Dissection Detection Risk Score, a Novel Guideline-Based Tool for Identification of Acute Aortic Dissection at Initial Presentation. Circulation. 2011;123(20):2213-2218. doi:10.1161/CIRCULATIONAHA.110.988568
- Nazerian P, Giachino F, Vanni S, et al. Diagnostic performance of the aortic dissection detection risk score in patients with suspected acute aortic dissection. Eur Heart J Acute Cardiovasc Care. 2014;3(4):373-381. doi:10.1177/2048872614527010
- Asha SE, Miers JW. A Systematic Review and Meta-analysis of D-dimer as a Rule-out Test for Suspected Acute Aortic Dissection. Ann Emerg Med. 2015;66(4):368-378. doi:10.1016/j.annemergmed.2015.02.013
- Nazerian P, Mueller C, Soeiro A de M, et al. Diagnostic Accuracy of the Aortic Dissection Detection Risk Score Plus D-Dimer for Acute Aortic Syndromes: The ADvISED Prospective Multicenter Study. Circulation. 2018;137(3):250-258. doi:10.1161/CIRCULATIONAHA.117.029457