Addressing the Controversies Around Thrombolytic Therapy (tPA)
In our previous blog post, we reviewed the formative literature supporting the currently used tPA time window. The figure below highlights a summary of the time windows investigated within each study and their respective findings (white lines indicating equivocal results, red for negative outcomes with tPA administration and green indicating a favourable outcome with tPA administration) with regards to thrombolytic therapy for the treatment of acute ischemic stroke.
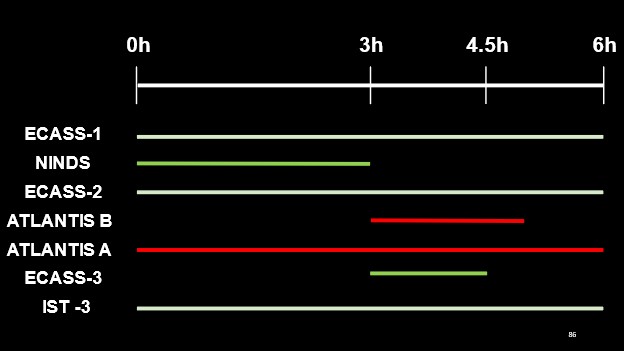
But what if someone came along and told you that both NINDS and ECASS-III were wrong? Would you still believe in tPA’s efficacy, if no trials had satisfied their primary outcomes?
The impetus for much of the concern for the use of tPA, comes from the Hoffman and Alper Re-Analyses of these two respective landmark trials (NINDS AND ECASS-III). [1,2] As a result of these studies, several arguments agains the use of tPA have been brought forward. This blog post, will attempt to address these.
ARGUMENT 1
“The Hoffman re-analysis invalidates the findings of the NINDS paper, as adjustment for baseline stroke severity leads to no difference as compared with placebo” [1]
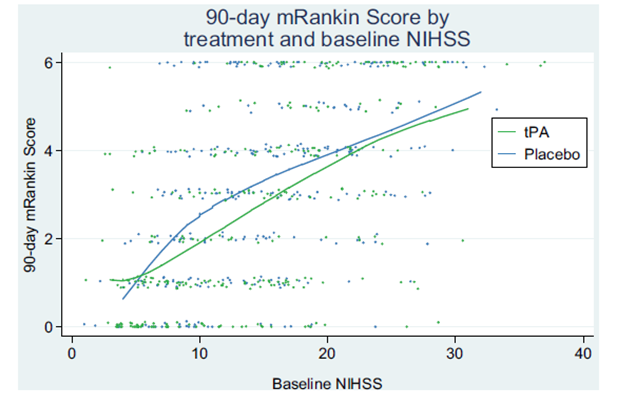
Figure 1: As Taken from Hoffman et al [1]. Qualitatively refutes tPAs benefit in the treatment of acute ischemic stroke.
The above figure is from the paper, and the authors posit that it shows qualitatively that there is NO BENEFIT for tPA when stroke severity is corrected for.
However, I would offer an alternative interpretation:
1. Across a spread of baseline stroke severities, tPA recipients had lower 90-day modified rankin scores. This supports the modest, but real, benefit of tPA.
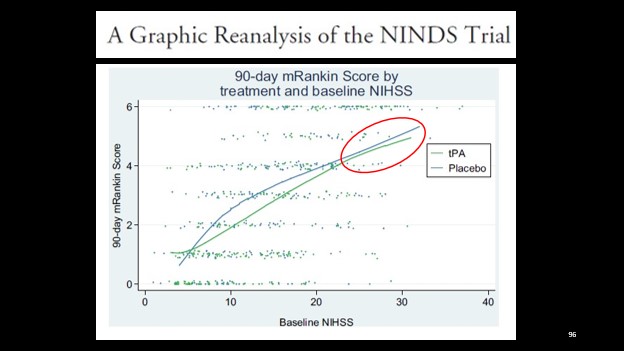
2. The difference is still present, but seems reduced in those with higher baseline NIH scores. This is consistent with our understanding of stroke physiology.
We know that higher stroke scores are frequently associated with more proximal thrombus – such as the internal carotid artery or proximal MCA strokes. We also know that from the work of Menon et al. in 2018, that these patients benefit less from tPA, whereas those with more distal MCA clots have much higher recanalization rates. [3]
Thankfully, now we have endovascular therapy as an available treatment method, for more proximal clots.
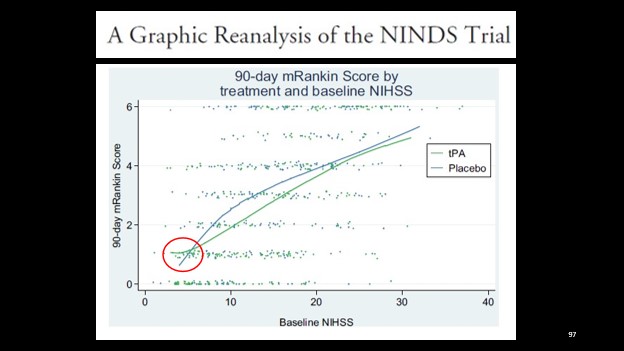
3. At the opposite end of the spectrum, we see one area where placebo seems to outperform tPA – patient with lower stroke severities.
This finding has also been borne out in the literature.
The PRISMS RCT looked at this exact question – in patients presenting within 3 hours with minor deficits, was Alteplase as compared to ASA beneficial?
The trial was stopped early due to slow recruitment leading to withdrawal of sponsor funds, however, the analysis of the data collected (n = 313) found a trend towards poorer neurological outcomes with tPA (not statistically significant), and an increased symptomatic intracranial hemorrhage rate of 3.2%. [4]
TEMPO-2, a Canadian study currently underway, looks to answer this question more definitively.
Thus, a more accurate interpretation of Figure 1 might be that the NINDS trial data re-analysis suggests that tPA is beneficial across a breadth of stroke severities, but that in those with less disabling strokes it is unclear if that benefit is maintained.
ARGUMENT 2
“Following adjustment for baseline characteristics in the NINDS trial the notion of the “TIME IS BRAIN” paradigm disappears”
Additionally, data from sub-group analyses of the IST-3 trial, by time category, further argues against this paradigm, calling into question the entire physiologic basis upon which thrombolysis time windows are based.
While these individual points are correct, I think an appropriate explanation is that neither of these studies were powered to answer these respective questions.
A 2010 meta-analysis of 3670 patients however finds a stepwise, statistically significant benefit to administration of tPA following onset of symptoms, with earlier administration being associated with better outcomes.
Statistically significant evidence of benefit is lost in the 4.5-6h time window. [5]
ARGUMENT 3
“The harms are too high, and outweigh any benefit”
This was a legitimate question and stance held by many prior to available data from multi-national registry data.
The findings of the SITS-MOST, CASES, and STARS registries are consistent with one another, and reassuring in this respect.
To highlight the findings of the SITS-MOST registry (the largest of these registries), as compared with the pooled placebo arms of the existing stroke RCTs, the sits most study found 15% improvement in rates of functional independence, a consistent rate of symptomatic ICH (~7%) and a mortality rate ~6% lower for recipients of tPA.
While comparison between the aggregate placebo arms of preceeding RCTs and registry data isn’t the most robust means of comparison, it does articulate and contextualize the above, meaningful data which highlights safety for tPA administered within 3 hours. [6]
ARGUMENT 4
“The Alper paper highlights vulnerabilities in the ECASS-III study, without which we have no basis for a 3-4.5h time window”
In adjusting for baseline imbalances between arms and re-examining the data using a number of statistical methods, statistical significance for benefit was lost in the majority of forms of analysis, while harm related outcomes were maintained.
While re-analysis of the data cannot change the trial outcome, they highlighted that it can change our certainty around it. [2]
This argument is well taken, and if the ECASS-III trial were the only data we had supporting a 4.5h time window, it would be absolutely imperative that we re-evaluate this. Fortunately, however this isn’t the case.
The best data we have on tPA is an aggregate of all existing RCT individual patient data published by Emberson et al. in the Lancet in 2014. This study of nearly 7000 patients found a statistically significant 5.2% absolute benefit for excellent neurological outcomes in those treated between 3 and 4.5 hours.
The benefits we offer those in this time group are modest, yet substantial. And while this therapy isn’t perfect, this meta-analysis found no difference in mortality in this group, and a 5.5% increase rate of symptomatic intracranial hemorrhage. [7]
Another popular meta-analysis, published by Donaldson et al. and favoured by some tPA skeptics, notably found tPA to provide patients with statistically significant increases in independence (OR 1.2 [95% CI 1.08-1.33], P = 0.001) and no difference in mortality (OR 1.04 [95% CI 0.92-1.18], P = 0.49). [8]
CONCLUDING STATEMENT
I hope that this exploration and interpretation of the evidence reassures you that when we administer this therapy in conjunction with our neurologist colleagues, we provide our patients a modest but real opportunity at retaining independence and do so with an acceptable safety profile.
[1] Hoffman JR, Schriger DL. A Graphic Reanalysis of the NINDS Trial. Ann Emerg Med 2009;54. https://doi.org/10.1016/j.annemergmed.2009.03.019. [2] Alper BS, Foster G, Thabane L, Rae-Grant A, Malone-Moses M, Manheimer E. Thrombolysis with alteplase 3-4.5 hours after acute ischaemic stroke: Trial reanalysis adjusted for baseline imbalances. BMJ Evidence-Based Med 2020;25:172–9. https://doi.org/10.1136/bmjebm-2020-111386. [3] Menon BK, Al-Ajlan FS, Najm M, Puig J, Castellanos M, Dowlatshahi D, et al. Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA – J Am Med Assoc 2018;320:1017–26. https://doi.org/10.1001/jama.2018.12498. [4] Khatri P, Kleindorfer DO, Devlin T, Sawyer RN, Starr M, Mejilla J, et al. Effect of alteplase vs aspirin on functional outcome for patients with acute ischemic stroke and minor nondisabling neurologic deficits the PRISMS randomized clinical trial. JAMA – J. Am. Med. Assoc., vol. 320, American Medical Association; 2018, p. 156–66. https://doi.org/10.1001/jama.2018.8496. [5] Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 2010;375:1695–703. https://doi.org/10.1016/S0140-6736(10)60491-6. [6] Toni D, Lorenzano S, Puca E, Prencipe M. The SITS-MOST registry. Neurol Sci 2006;27. https://doi.org/10.1007/s10072-006-0632-9. [7] Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–35. https://doi.org/10.1016/S0140-6736(14)60584-5. [8] Donaldson L, Fitzgerald E, Flower O, Delaney A. Review article: Why is there still a debate regarding the safety and efficacy of intravenous thrombolysis in the management of presumed acute ischaemic stroke? A systematic review and meta-analysis. Emerg Med Australas 2016;28:496–510. https://doi.org/10.1111/1742-6723.12653. REFERENCES



Thanks for the articles Dr. Drew. This ongoing discussion, although somewhat tedious, is really important as we all strive to provide our patients with the best possible care.
I think there are some problems with the way you framed these articles. Most importantly, I don’t think anyone is actually making any of the 4 arguments that you rebutted here, and therefore they are probably straw men.
I wrote a response that covers my thoughts on my blog, and it can be found here: https://first10em.com/the-ongoing-thrombolytics-debate-a-response/
All the best
Justin