Do you remember when every newborn under 30 days with a fever needed an LP/admission/blood cultures? Well.. times are a little different but its nuanced. The goal of this update is to provide a practical update in pediatric emergency medicine to the average Canadian adult or community emergency clinician. We will review the following three topics:
- 2021 American Academy of Pediatrics guidelines for the febrile infant1
- Key updates in pediatric DKA management as per 2023 TREKK guidelines2
- Key updates in pediatric status epilepticus management as per the 2022 TREKK guidelines3
By the end of this post, you will be well equipped to care for critical pediatric presentations, regardless of how often you see children in your practice.
2021 American Academy of Pediatrics guidelines for the febrile infant: 8-60 days
History of the Febrile Infant
Countless efforts have been made since the 1970s to generate an evidence-based practice guideline for the management of the febrile infant. This stemmed from the concern around the rapid progression of Group B strep infections in neonates, which could present with a clinical appearance and laboratory results that did not reflect the seriousness of the disease. For a while, all febrile infants under the age of 60-90 days, depending on the institution, were hospitalized and underwent extensive investigations and treatment with broad-spectrum antibiotics.4 By the 1980s, evidence started to emerge around the harms and costs of unnecessary hospitalizations and testing. This lead to the development of multiple prediction models focused on detecting serious bacterial illnesses (SBIs) and later, the prediction models for low-risk infants. These rules relied heavily on clinical parameters and arbitrary lab values. For example, the use of WBC 5-15 in many of the low-risk prediction models was chosen a priori, and never statistically derived as a reassuring feature. Over the years, there has been significant heterogeneity in practice patterns across major pediatric referral centers and between individual pediatric emergency providers.
Less is more?
New guidelines were felt to be needed for several reasons. First, there is evidence suggesting that physicians are not actually very good at following the older guidelines, and that failure to follow guidelines did not necessarily result in worse outcomes in certain cases.5 Second, there’s been a significant change in the bacterial landscape since the 1980’s and 90s when many risk stratification tools were developed. This is due to prenatal GBS screening, increased food safety and widespread vaccination against streptococcus pneumoniae. Currently, E. coli has overtaken GBS as the most common organism causing bacteremia in infants (although most studies still note GBS as the most common cause of meningitis). Finally, there are huge costs associated with unnecessary care, both in dollar amounts and iatrogenic insults from hospital-acquired infections.
Now, time to get in to selected key details of the 2021 AAP guidelines.
1. Development of the Guideline
The guidelines were developed by a working group consisting of representatives from (but not limited to) epidemiology, general pediatrics, pediatric emergency, pediatric subspecialties, infectious disease, along with individuals with expertise in guideline development, algorithm creation and quality improvement. Formal analysis and systematic review were performed, followed by a secondary analysis by the epidemiologists. The group then solicited data from known authors and working groups in attempts to fill any gaps in the literature. After the development of the recommendations, they were reviewed by multiple physician groups as well as focus groups of parents.
2. Goal of the Guideline
To improve the diagnosis and treatment of UTIs, bacteremia and meningitis (serious bacterial illnesses).
3. Inclusion Criteria
- Well appearing
- T ≥ 38 rectal
- 37-42w gestation
- From home
4. Exclusion Criteria
- Age <8 days
- Premature (born under 37 weeks)
- Age < 2 weeks where mom had peripartum fever, infection or antimicrobial use
- High suspicion of HSV (lesions present)
- Obvious focal bacterial infections such as cellulitis or septic arthritis
- Immune compromised or medically complex, which is further outlined in detail in the guidelines
- Notably- guidelines excluded patients with bronchiolitis
- Neonatal surgery or infection
- Congenital or chromosomal abnormalities
- “Medically fragile” children, defined as requiring technology or therapy to sustain life
- Immunized in last 48 hours (40% will have fever > 38 within first 48h)
5. Role of Viral Testing
While multiple studies have shown lower rates of invasive bacterial infections in subsets of patients with positive viral testing, The AAP states “rapid molecular testing is becoming available at a rate that is outpacing evidence for how this testing should be used”. No prospective study has provided convincing data on whether a positive viral test result reduces the infection risk enough to change decision making. So for now, a positive viral test, especially in age <28d, should not alter our decision making. Bronchiolitis on the other hand CAN be excluded from this pathway based on a review of 11 studies that showed no cases of meningitis in infants with bronchiolitis.6-9
6. Inflammatory Markers (IM)
To assess inflammatory markers, there are two scenarios used depending on whether your hospital has access to procalcitonin testing or not.
- If procalcitonin is available, you should use procalcitonin + either ANC or CRP as your IM assessment. Both markers need to be negative for your IM assessment to be “negative,” but only one needs to be above threshold value to be considered “positive.”
- If procalcitonin is not available, you should use temperature >38.5C, ANC and CRP as your IM assessment. Elevation of any one of the three is considered “positive.”
The cutoff values for elevation are:
- Procalcitonin >0.5mg/mL
- CRP >20mg/L
- ANC >4000/mm3
- Temperature > 38.5C
You are likely familiar with all of the above markers except procalcitonin. So what is it?
Procalcitonin is expressed by thyroid C cells and is produced rapidly in response to infection. It has emerged as the most accurate inflammatory marker for risk stratification, rises more rapidly and is more specific to bacterial infections than other inflammatory markers such as CRP. However, no single inflammatory marker is good enough to stand alone. Even procalcitonin, which outperforms all the others, is inadequate in isolation, which is why it is used in combination with others in this guideline. In the step-by-step validation study in 2016, 20% of febrile infants with bacterial meningitis had procalcitonin levels <0.5ng/ml.10
7. Guideline: Infants age 8-21 days
- Urinalysis
- Urine culture
- Blood culture
- Lumbar puncture
These patients are getting the full septic workup including urinalysis, urine culture, blood culture the lumbar puncture, similar to what we always used to do for most infants under age 1 month. These patients all need admission to hospital and IV antibiotics until cultures come back negative. The rate of bacteremia in the 8-21d group is estimated between 4-5%, and even up to 2% in patients with negative urinalysis and negative inflammatory markers, justifying the broad workup and treatment strategy in this age group. Inflammatory markers can be drawn, but do not alter decision making in the moment. Some physicians report that it helps decision making around antibiotics at the 24-36h mark or while awaiting final cultures.
Empiric antibiotic regimens will be tailored by your local pharmacists and antibiograms, but all protocols are built on the notion that there is good coverage for GBS, gram negatives, and have adequate CNS penetration in cases of suspected meningitis. The AAP recommends ampicillin and gentamycin for unknown source. If meningitis is suspected based on CSF results, the recommended antibiotics are ampicillin and ceftazidime.
8. Guideline: Infants age 22-28 days
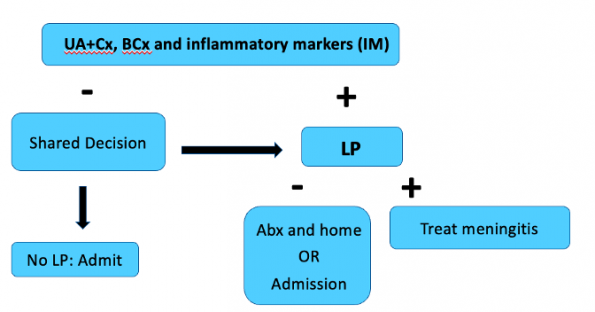
*Flowchart adapted from AAP Guidelines
Moving on to the 22-28 day group, the first step is to obtain a urinalysis and culture, blood cultures and inflammatory markers. Even if the urine is positive, we need to continue with the assessment as there is insufficient evidence in this age group to estimate the risk of meningitis in the case of a positive urine. If inflammatory markers are positive, an LP is indicated. CSF results suggestive of meningitis will get admitted with IV antibiotics. If the lumbar puncture is negative, there are two options:
- Receive 1 dose parenteral antibiotics, discharge home, and have follow up in 24 hours
- Admission to hospital for monitoring with or without antibiotics
If inflammatory markers are negative, you can engage in shared decision making based on your risk tolerance and parental preference on whether or not to pursue a lumbar puncture. If no LP is done (or if the LP is insufficient/uninterpretable), the patient needs to be admitted for monitoring either with or without antibiotics. If the LP is done, they re-enter the pathway as previously discussed.
I want to take a moment to highlight one of the biggest practice changes that comes from these guidelines:
Well appearing infants age 22-28d with normal urinalysis and inflammatory markers MAY OPT OUT of a lumbar puncture based on shared decision making between the physician and parents.
The AAP’s recommendation for empiric antibiotics in this age group includes ceftriaxone if the source is unknown, and ampicillin/ceftazidime if the CSF is suggestive of meningitis.
Another big update in this guideline is that in infants age >21 days, a bag-collected urine sample is sufficient to rule out UTI. Any positive samples will need to be confirmed with a catheter-obtained sample, but we are now able to use a bag urine as a “rule out test.” This can avoid iatrogenic infection introduced by catheterization, and is much easier- especially at non-pediatric hospitals where your colleagues might not be used to catheterizing a 3 week old infant.
9 Guideline: Infants age 29-60 days
We’re not going to review the 29-60 day algorithm in detail today, as most of the principals of the decision making are the same as the 22-29d infants. There is a LOT of room for shared decision making in this age group. The only firm recommendations are that
- Negative inflammatory markers can always go home.
- If they have a UTI and negative inflammatory markers, treat them with PO antibiotics.
- If normal inflammatory markers and no UTI, the recommendation is discharge home with no antibiotics, with clinical reassessment in 1-2 days.
The rest of the 29-60d algorithm has a ton of options that we’re not going to discuss in detail. I recommend contacting your local pediatric referral center for assistance in shared decision making.
10. Suspected HSV infection
If the patient has RF or signs of HSV, you also need to add acyclovir. The guidelines indicate that acyclovir should be added when:
- Maternal lesions or fever from 48h before delivery to 48h post-partum
- Infants with vesicles or mucous membrane ulcers
- Hypothermia
- CSF pleocytosis (elevated cell count) with no gram stain result
- Leukopenia
- Thrombocytopenia
- Elevated ALT
11. Shared Decision for LP
I keep referring to this option of shared decision making with the parent. I’m sure many of you are thinking- that sounds great, but how do we actually counsel parents around the LP decision? Well, the AAP network is generating a “parent engagement toolkit” with step-by-step guides and even scripts that physicians can follow to help with this decision making. To assist you with engaging in this conversation, some important numbers include the 0.1% risk of serious complications from an LP (bleeding, infection or nerve injury). They also quote a 0.1% risk of meningitis in this group of infants (those age 22-28d with normal inflammatory markers).
Summary of Major Practices Changes from the AAP 2021 Guidelines:
- There is now a younger age cutoff for mandatory full septic workup including LP (age 21 days).
- Well-appearing infants age 22-28d with normal urinalysis and inflammatory markers MAY OPT OUT of a lumbar puncture based on shared decision-making between the physician and parents.
- Inflammatory markers including procalcitonin are key to risk stratification in all age groups.
- Infants >21d old can give a urine sample via bag-collection method rather than catheterization.
Diabetic Ketoacidosis: 2023 Update to TREKK Guideline2
Update: All pediatric DKA patients should receive a 20ml/kg fluid bolus up front.
The last 5-10 years has shown great controversy and debate around the pathophysiology of cerebral injury in the context of DKA. The previous doctrine was that fluid itself is a problem- that fluid administration created higher risk for cerebral edema. There is now evidence showing that cerebral perfusion and a hyperinflammatory state caused by DKA are likely playing the most important roles in development of cerebral injury, and that variations in fluid treatment have minimal effect. The concern around fluids was that rapid changes in serum osmolality were leading to cerebral edema, but more recent evidence suggests that osmolality and osmotic changes during treatment have actually not had any correlation with the degree of cerebral edema development.
You’ll also notice that the guidelines now use the phrasing, “cerebral injury” rather than “cerebral edema.” The reason for this is that recent neuroimaging studies have shown that subclinical cerebral edema is occurring much more frequently than we thought. Cerebral injury is the phrase that is now used when discussing the clinically apparent cerebral edema, meaning the cases of cerebral edema that result in end organ damage. In other words, cerebral injury is used to describe the cerebral edema that we care about clinically.
The new recommendations are largely based on the 2018 PECARN fluid trial.11 This was a randomized controlled trial that sought to assess the ideal rate of fluid resuscitation, as well as the sodium chloride content of the resuscitation fluid. It was a 2×2 factorial design RCT, that included 1389 incidences of DKA. The rapid fluid administration group received 2 boluses, each 10ml/kg (so 20ml/kg bolus total) followed by half their fluid deficit replaced in 12 hours, and the remaining deficit replaced over the subsequent 24h. The slow fluid administration group received a 10ml/kg bolus followed by the calculated fluid deficit being replaced evenly over 48h.
The primary outcome was decline in GCS to less than 14, and the study found no difference in this primary outcome between any of the trial arms.
This trial largely forms the basis for the TREKK and ISPAD guidelines to give up to two 20ml/kg boluses, and then initiate fluid infusions as per the weight based chart in the TREKK guideline. The weight based chart is built to replace a 10% fluid deficit over 36 hours, which is in keeping with the rapid fluid replacement arm of the study. We know these patients are hypovolemic, and now have high quality evidence that proper fluid replacement is safe, so don’t hesitate to resuscitate your patient!
On that note, one limitation of the PECARN fluid study is that there was not a high proportion of really sick DKA patients, so it’s appropriate to acknowledge that our evidence in treating the super sick peds DKA is still not as robust as we would like.
Our local pediatric emergency physicians recommended a few key pointers when managing the pediatric DKA patient:
- Get on the phone with your peds referral center EARLY. They will help you provide nuanced care to the patient in front of you
- The neurologic assessment is KEY! Look up the pediatric GCS in every case and make sure you are applying it properly (don’t rely on your memory, the pediatric GCS changes between multiple age groups). Pay close attention to irritability in the pre-verbal child and assess how they are responding to their caregiver. Be sure to convey the neurologic status as accurately as possible to the referral center so that you can get appropriate recommendations regarding cerebral injury management.
As you know, treat any suspected cases of cerebral injury by elevating the head of bed, ensuring adequate perfusion, and treating with hypertonic saline or mannitol. You should not delay transport to your pediatric referral center for a CT scan.
You’ll notice that there is still some lingering concern from the old days around fluids and cerebral injury in the TREKK guidelines. For example- they do recommend reducing the fluid infusion rate to 75% in patients who DO have apparent cerebral injury. This demonstrates that there is a group of experts who are still hesitant to completely discount the effect of fluids on cerebral injury. For now, I recommend following this guideline, understanding that it’s not necessarily grounded in evidence.
Status Epilepticus: 2022 Update to TREKK Guideline3
Finally, we are going to touch on status epilepticus. The key update in the 2022 TREKK guideline reflects recent studies comparing second-line antiepileptic agents. So we’re talking here about which agent to use in your status epilepticus patient that is refractory to 2 doses of benzodiazepines. Remember, you are calling for your second-line antiepileptic at the same time as your second dose of benzodiazepines.
The 2019 TREKK guidelines had 3 choices of second-line agents to use. Fosphenytoin, the phenytoin prodrug, was a long-time favourite option. Compared to phenytoin, fosphenytoin could be given faster and also had less local tissue toxicity at the injection site. Phenytoin or fosphenytoin was recommended as the favourite second-line option based on observational study and expert opinion only, with no high-quality RCT evidence. Based on several studies that we are going to discuss, the options have been expanded to include Levateracitam (Keppra) and valproate.
Many of you will recall that phenytoin/fosphenytoin are almost always on your list of drugs that can cause horrible side effects, including SJS, hepatotoxicity, pancytopenia, local extravasation tissue injury, hypotension and cardiac arrhythmias. Given the favourable side effect profile of Levetiracetam, there has been considerable effort to assess if it is better than, or at least as good as phenytoin for seizure cessation.
The first two trials we will review are ConSEPT and EcLiPSE.12-13
The ConSEPT trial was published in 2019. This was an open label, multicenter RCT conducted in 13 emergency departments in Australia and New Zealand. Its goal was to determine whether or not phenytoin or levetiracetam was the superior second line treatment for status epilepticus in children. It enrolled specifically pediatric patients between the age of 3 months and 16 years who had failed first line antiepileptic treatment (benzodiazepines). They had 233 children enrolled in the study. They excluded patients who were on one of the study drugs at baseline at home. This study compared phenytoin 20mg/kg with levetiracetam 40mg/kg. The primary outcomes was seizure cessation 5 mins after the end of the infusion. The results showed seizure cessation in 60% of the phenytoin group and 50% of the Keppra group, the difference was not statistically significant. In summary- this open-label study did not show superiority for levetiracetam, it showed no difference between treatment groups of any of its efficacy or safety outcomes. EcLiPSE was also an open label superiority trial between phenytoin and levetiracetam, done in 30 pediatric emergency departments in the UK. It used the same doses as in the ConSEPT trial. It included 286 participants between the ages of 6 months to just under 18 years. It too found that levetiracetam was not statistically superior to phenytoin, but suggested that it could be an alternative to phenytoin In second line management of pediatric convulsive status. The main trial that is quoted as the basis for the TREKK update is the ESETT trial. This was a double blind, response adaptive randomized controlled trial published in the Lancet in 2020. This is currently the highest quality evidence we have for second line anti-epileptics in both adult and pediatric patients. This trial enrolled patients age 2 or older, who had been treated for generalized convulsive seizure longer than 5 minutes, with adequate doses of benzodiazepines. It included 462 patients of all ages: 225 children, 186 adults age 65 and under and 51 adults over age 65. They compared levetiracetam, fosphenytoin and valproate, with the primary composite outcome of “absence of clinically apparent seizures with improving responsiveness at 60 minutes after the start of drug infusion.” Secondary outcomes included intubation, seizure recurrence within 12h, respiratory depression and mortality. I want to highlight that this study used Levetiracetam dosing of 60mg/kg, which is also now the recommended dosing by the American epileptic society. This is an increase from previous trials. The previous two trials we talked about (Eclipse and Consept), were open label, citing difficulties in blinding the medications due to differences in infusion times. This study was blinded, and just infused each drug over 10 minutes. They had vials that were identical, and each vial came with an assistive device that had pre-programmed infusion rates and could allow for unmasking of the drug if required by the treatment team. Rescue medication could be provided at the 20 minute mark if needed, and unmasking was allowed at 60 minutes if it was necessary to know which drug they had received. The overall trial was stopped early at a planned interim analysis of 400pts for futility, but continued to enroll pediatric patients until they had sufficient power to complete their secondary age based analyses. The ESETT trial found no difference between the three agents in primary outcome of seizure cessation at 60 min, with a cessation rate of approximately 50% for all treatments. It also did not detect any differences in primary safety outcome (composite of hypotension or life threatening cardia arrhythmia). It did find a difference in intubation ratesat 60 minutes- which was one of its secondary outcomes (33% intubation rates in the fosphenytoin group, 8% in Leviracetam and 11% in valproate). The authors recommend interpreting secondary outcomes with caution as these results had not been shown in any of the previous studies, and the study was not appropriately powered for their secondary outcomes. Furthermore, they didn’t see a difference in rates of respiratory depression, so it’s not entirely clear why the intubation rates differed. However at the end of the day, the goal is to stop the seizure as fast as possible, so the best second line agent is whatever can be administered the fastest. If your center doesn’t have levetiracetam stocked in the ED, it is likely not your best choice. I encourage you to familiarize yourself with your department’s access to variable second line antiepileptics. There is absolutely lots of variety in recommendations and nuance, particularly in patients who present with known seizure disorders and those who are already on antiepileptics at home. Neurologists may recommend an alternate agent or loading dose adjustments. All of these decisions require consultation with pediatric emergency or neurology specialists, which can be challenging to arrange from an outside center at the speed necessary to treat status epilepticus. With these considerations and the time constraints in mind, I recommend the following strategy to your second line antiepileptics: ConSEPT
EcLiPSE
ESETT Trial14
All of this has lead to the 2022 TREKK recommendation that any of the listed agents (levetiracetam, fosphenytoin, phenytoin, valproate or phenobarbital) can be used for second line treatments. There is a preference for Levetiracetam if available given an improved side effect profile, few drug interactions and the ability to provide it over 5 minutes.
References


