Point-of-care ultrasound (PoCUS) is becoming a cornerstone tool in our assessment of patient presentations and is helping us guide our clinical management. This is also represented in the amount of literature that has been published on the use of PoCUS in 2023. In this post, Dr. Murray reviews 5 articles (with case examples and videos), that he believes will be the most impactful on our practice and provides some pearls on how to implement them.
Femoral Artery Peak Systolic Velocity During Pulse Check in Cardiac Arrest
A 55-year-old male with a witnessed out-of-hospital cardiac arrest presents with CPR ongoing. At the first pulse and rhythm check you try and feel for a pulse, a second MD is looking at the heart in the subxiphoid region.
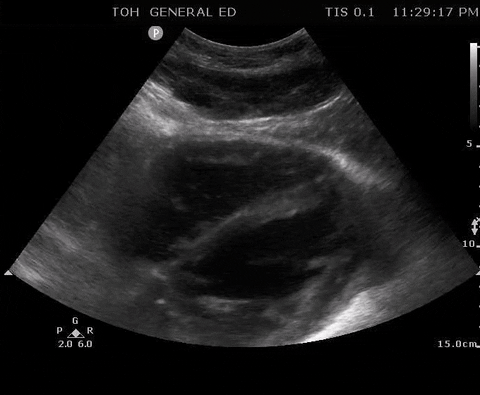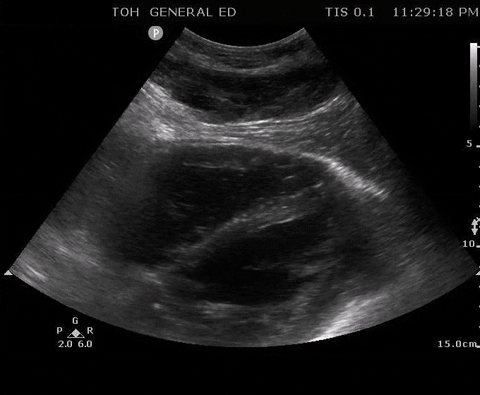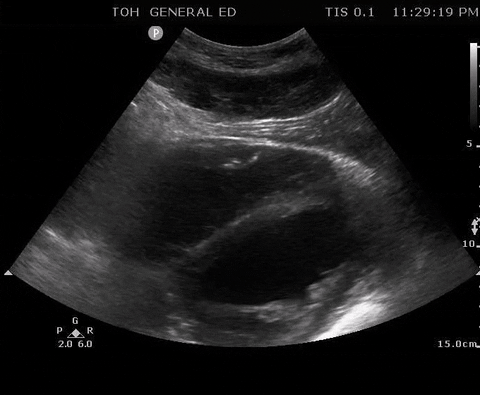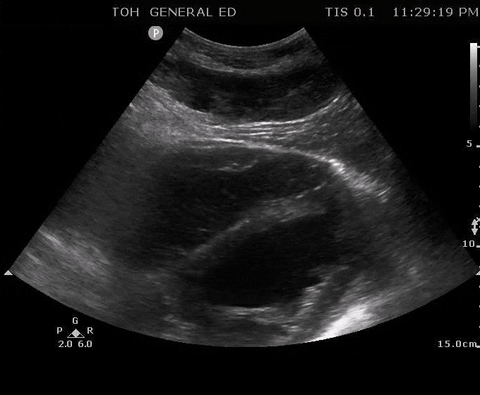
Subxyphoid PoCUS
Despite your best efforts, the only pulse you feel is your own. Your end-tidal CO2 monitor is being finicky, and you wonder if this patient has ROSC. You resume CPR and wonder what is the best way to check your patient’s pulse at the next pulse check?
Femoral Peak Systolic Velocity (PSV)
Using your ultrasound machine you can center your probe over the femoral artery and determine if a femoral pulse is present rather than relying on manual pulse checks.
Technique
- Identify the femoral artery using whichever probe you prefer
- Turn the Doppler mode on and centre the pulse wave gate over the femoral artery
- Start the pulse wave Doppler function
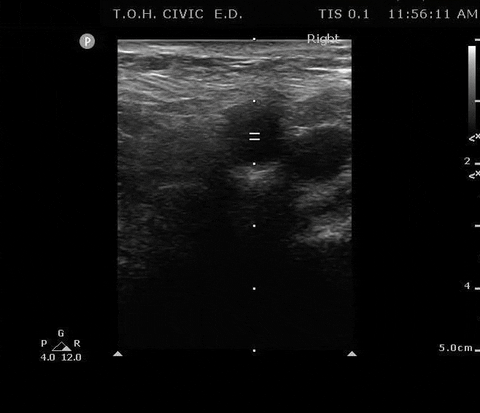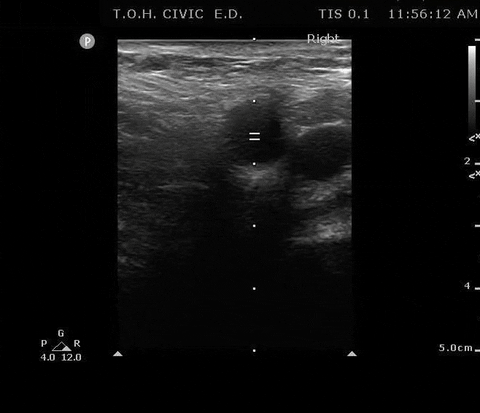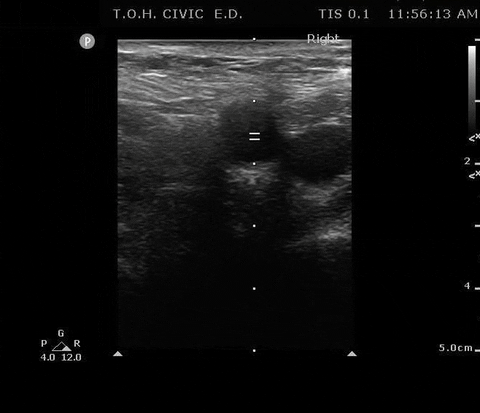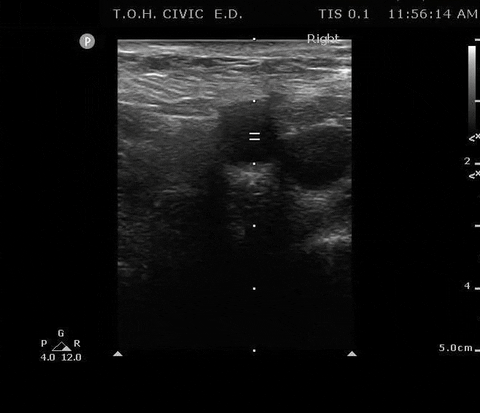
PSV femoral PoCUS
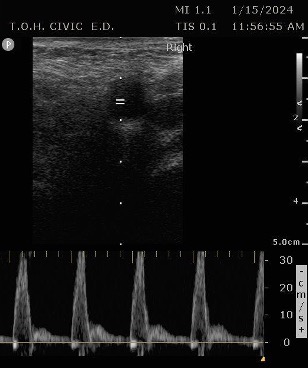
The peak measurement is the PSV measurement
Analysis
This was the secondary analysis of a previous study among ED cardiac arrest patients that defined ROSC as an SBP ≥ 60 mmHG. They found that femoral artery Doppler ultrasound was more accurate than manual palpation for detecting any pulse. When using a PSV ≥ 20 cm/s, Doppler ultrasound was also more accurate for detecting ROSC1.
Population
Patients in this study were >18 years old with OHCA or IHCA. Patients were mechanically ventilated with a femoral line in place. PSV was done by an EM doctor who was trained in the use of PW Doppler.
Methods
There was a concurrent recording of the highest SBP on the arterial line, and the highest PSV on Doppler and ETCO22.
- primary outcome: which correlation was better: PSV->SBP vs ETOC2->SBP.
- secondary outcome: whether PSV≥ 20cm/sec vs ETCO2 ≥ 20 or 25 mmHG more accurately predicted ROSC (SBP ≥ 60 mmHG).
Why ETCO2>20? The AHA recommends ETCO2 targets of 10-20 mmHG as an indicator of high-quality CPR. Recent literature has shown ETCo2 of >20-25 to be consistent with ROSC2,3. A recent 2021 study by Cricker et al. found the association ETCO2 > 20 mmHg being highly specific for ROSC in PEA patients4.
Results
A total of 35 pts and 111 pulse checks were analyzed.
PSV is better correlated with SBP than ETCO2 and SBP. The difference was found to be statistically significant. In terms of test characteristics, Doppler ultrasound using a PSV ≥ 20 cm/s, outperformed ETCO2 ≥ 20 mmHg, in the detection of ROSC for all test characteristics. When an ETCO2 cut-off of ≥ 25 mmHg was used the specificity (or ability to rule in ROSC) was the same as Doppler ultrasound (86%). Although the PPV and Positive likelihood for PSV and ETCO2 of ≥ 25 mmHg had different numerical values, the differences were not found to be statistically significant.
Strengths
- The treating team was blinded to ultrasound results.
- Rigorous statistical analysis with provision of test characteristics
Limitations
- Doppler ultrasound trained providers.
- Contradicts previous studies in main 3 ways:
- Higher rates of ROSC
- Potentially pts whom providers placed a line, intubated may have had better odds of surviving in general compared to others.
- CO2 was recorded at the end of pulse checks.
- Previous studies would check CO2 during CPR
- Higher proportion of PEA, previous studies had higher rates of shockable rhythms (43% were PEA vs 14% shockable)
- PSV trends may be different depending on PEA vs shockable rhythm (did not run separate analysis)
- PEA tends to be associated with pulmonary pathology that has the potential to influence ETCO2 readings.
- Higher rates of ROSC
Dr. Murray’s Recommendation
- Femoral artery PSV should not replace ETCO2 for determination of ROSC.
- PSV is a better alternative to manual pulse checks given the latter has poor evidence for reliability.
PoCUS Evaluation of IVC for Fluid Status Prediction
A 54-year-old female presents with hypotension and shortness of breath. You decide to do a PoCUS rush exam and this is your view of the IVC.
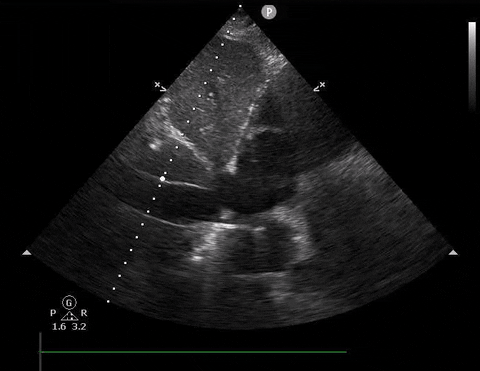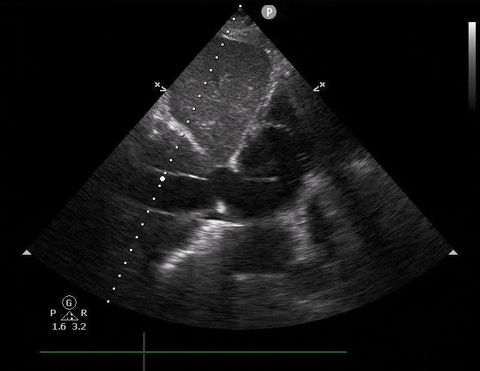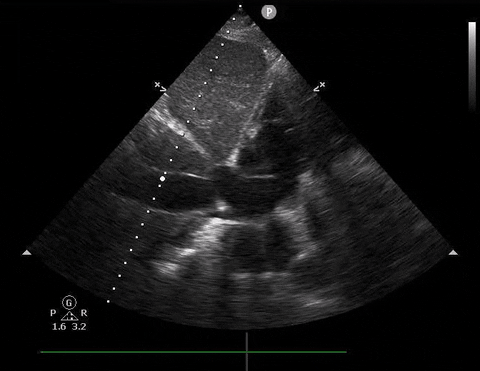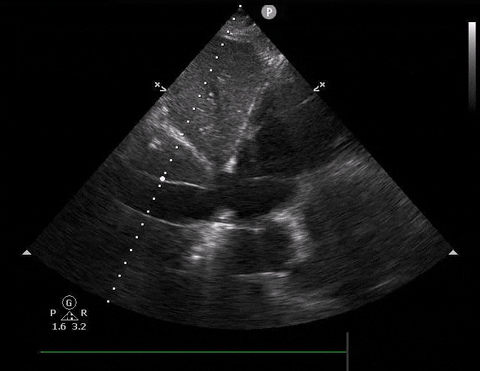
IVC PoCUS
Based on the image, should you give this patient a fluid bolus?
Context
Previous studies looked at IVC in mechanically ventilated patients and the evidence of using IVC to guide fluid administration has not survived the test of time.
Methods
This article was based on the multi-center international SHOCK – ED RCT (which is one of the most rigorous PoCUS articles ever done):5 Spontaneous breathing patients in shock were randomized to early point-of-care ultrasonography plus standard care versus standard care alone. The Shock IVC trial was a post-hoc secondary analysis of the data from the SHOCK-ED trial.
There were 138 spontaneously breathing patients randomized to the PoCUS group,
The authors collected data on IVC size and collapsibility and independently assigned fluid status determination from a blinded chart review which was used as the reference standard.
- primary outcome: if IVC ultrasound measurements of size and collapsibility could identify patients in shock who are volume overloaded at initial presentation6
Results
125 patients randomized to the POCUS group had both IVC scans and sufficient chart data to determine fluid status so were included in the final analysis.
When they looked at POCUS findings separately, they found the following:
- Dilated IVC >2.5 cm: sensitivity of 100% (95% CI 59.0 to 100.0%), and a specificity of 95.4 (95% CI 88.6 to 98.7%) for predicting fluid overload.
- Collapsibility was a less reliable independent measure: sensitivity of 85.2% (42.1 to 99.6%) and a specificity of 47.1% (36.3 to 56.1%)
They then combined size and collapsibility and found that the 2 combinations with the highest specificities were a normal IVC with no collapse or a dilated IVC with no collapse.
Strengths
- Results based on a multi-center international randomized control trial.
- Fluid volume status determination was done blinded to the ultrasound results.
- Primary outcome targeted at highest risk patients.
Limitations
- Retrospective
- Underpowered
- power calculation determined they would need 100pts with 20% of them not needing fluid administration. Only 16% were euvolemic or hypervolemic (<20% of patients did not need fluid administration)
- Did not report inter-rater reliability of volume status assessors.
- important as there is no “gold standard” for fluid volume assessment.
Dr. Murray’s Recommendation
- If your PoCUS shows an IVC >2.5cm or an IVC 1-2.5cm with no collapse, and your patient has no clinical findings of hypovolemia, then strongly reconsider the use of fluids to correct hypotension
Cardiac PoCUS to Differentiate Pulmonary Embolism and Chronic Pulmonary Hypertension
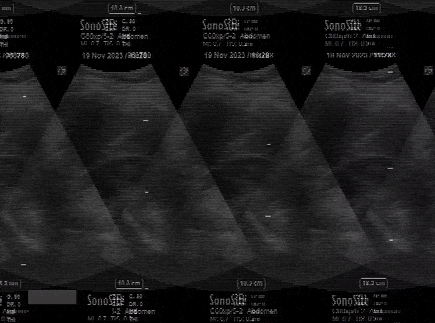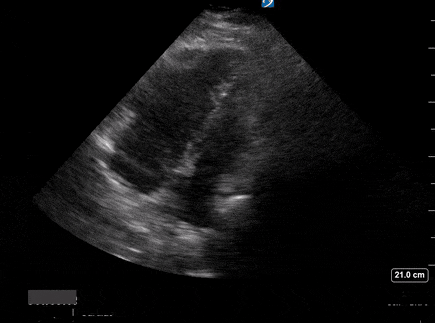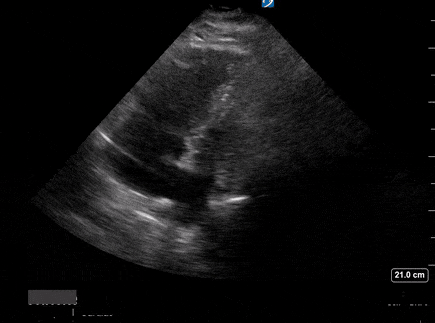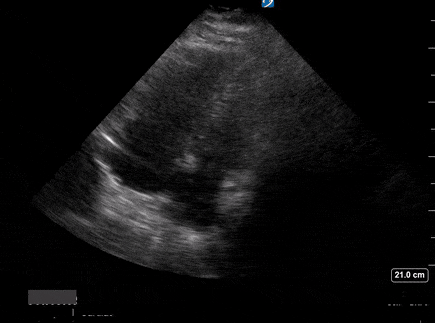
A 65-year-old male with a past medical history of COPD presents to the ER with pleuritic chest pain, hypoxia, and hypotension. You astutely grab your PoCUS machine to perform a cardiac exam and get the following images.
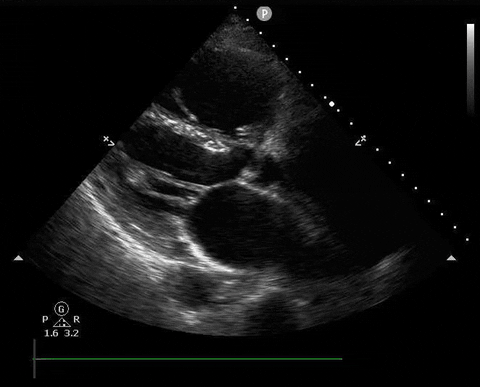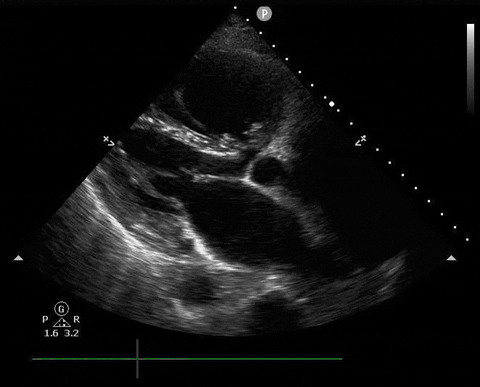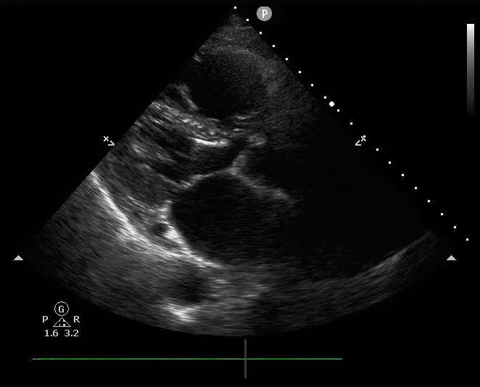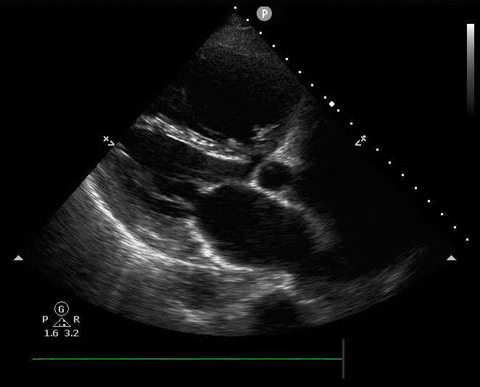
Parasternal long PoCUS
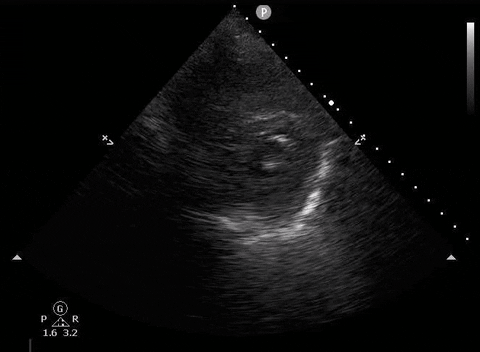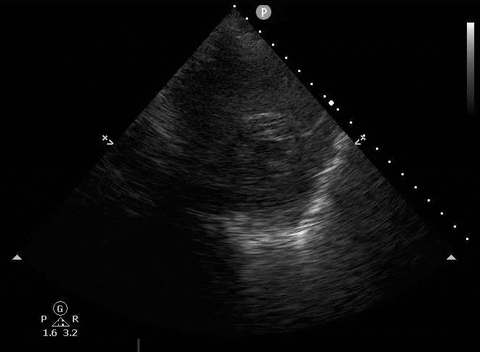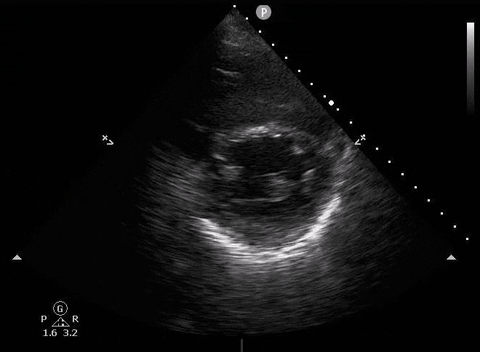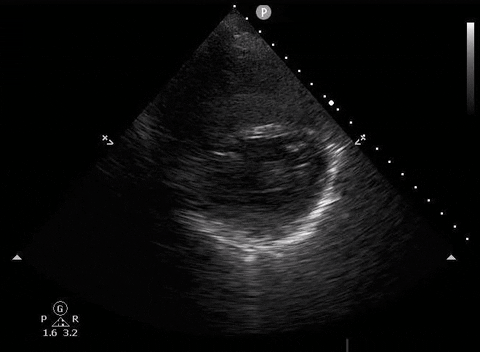
Parasternal short PoCUS
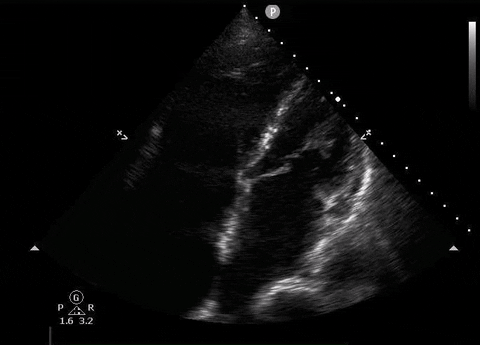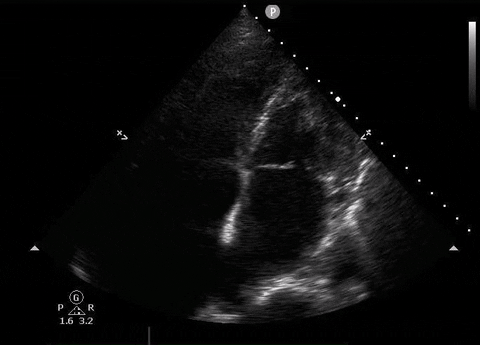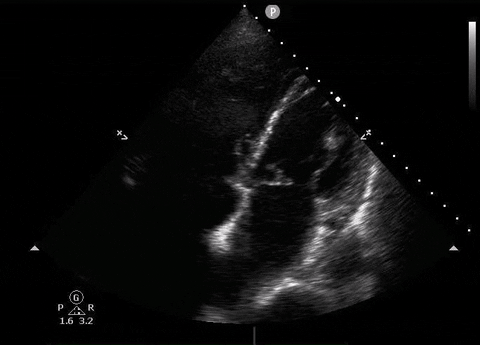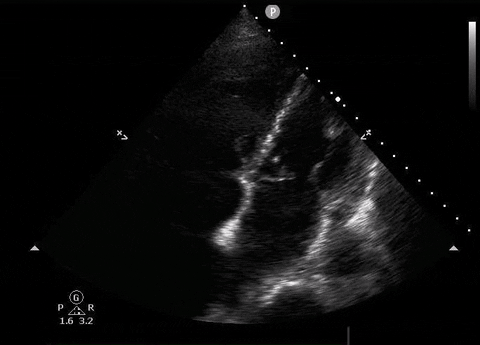
Apical four-chamber PoCUS
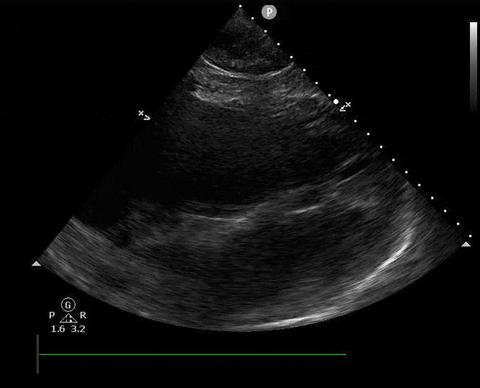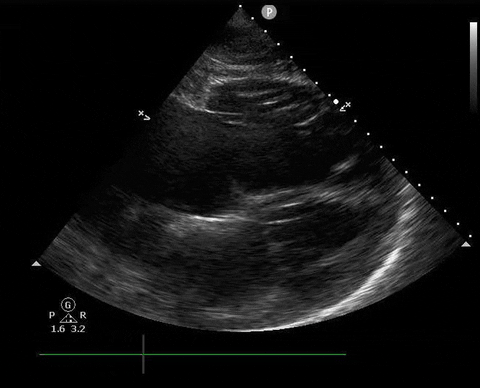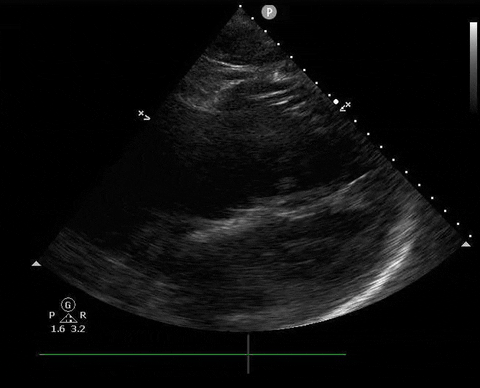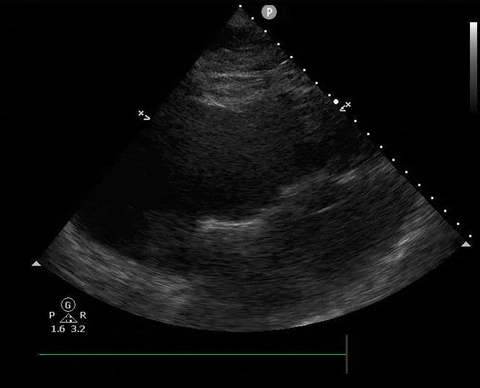
Does this patient have a PE? Or are the findings due to chronic pulmonary hypertension secondary to COPD?
Methods
This was a narrative review of the literature. The authors performed an Extensive search strategy of PubMed and Google Scholar using terms such as: “right ventricular dysfunction”, “right ventricular strain”, “right ventricle”, “”echocardiography”, “point-of-care ultrasound”, “POCUS”, “cardiac POCUS”, pulmonary embolism”, “pulmonary hypertension”, “cor pulmonale”, and each of the echocardiographic parameters. They also looked at the references as well of the articles to look for new articles. English language studies were selected by independent analysis and then consensus.
Inclusion Criteria
- Inclusion of pathophysiology
- Reference cut-off values
- Data for a given echocardiographic parameter.
Preferentially selected
- RCTs
- Prospective studies
- Retrospective studies
- Reviews
Strengths
- The best summary of evidence
- 175 references with priority on the highest level of evidence
- Extensive search with independent analysis
Limitations
- Like most narrative reviews it is limited by publication bias
- Limited to English language articles
Results
The authors looked at 8 findings you can see with POCUS. We will only be discussing the 4 that are within the scope of basic ultrasound skills.
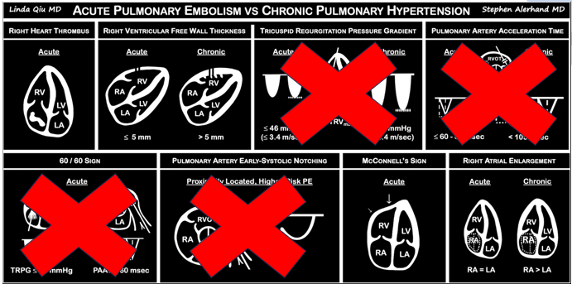
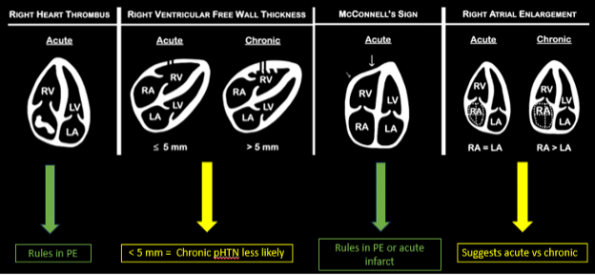
Presence of a right heart thrombus
A right heart thrombus appears as a long thin, free-floating wormlike mass. It is differentiated from vegetation or tumour in that it is not attached to the valve.
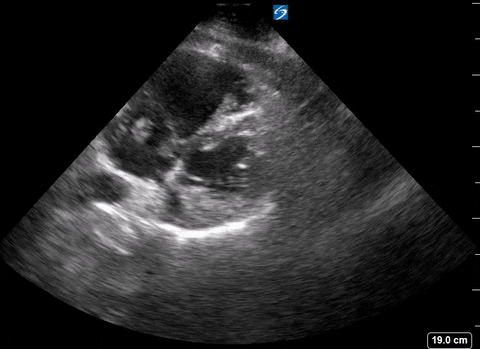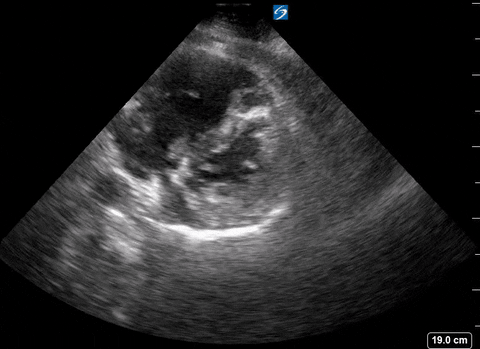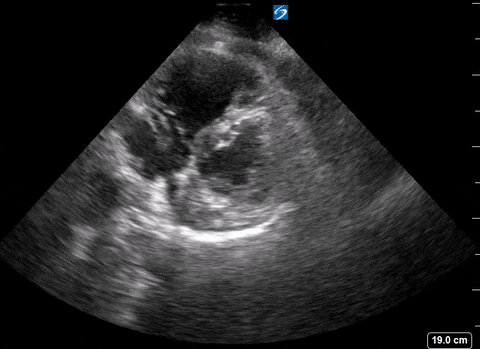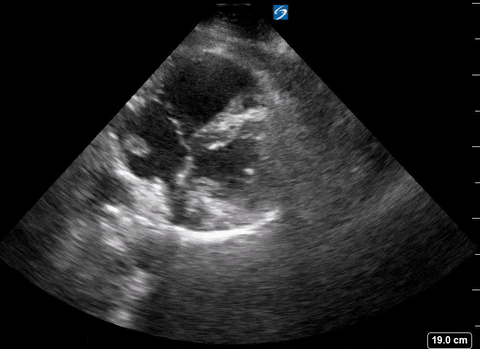
Right heart thrombus
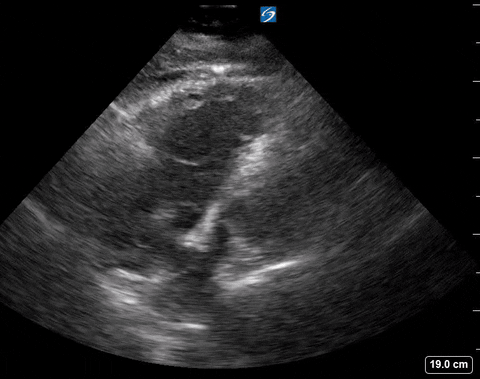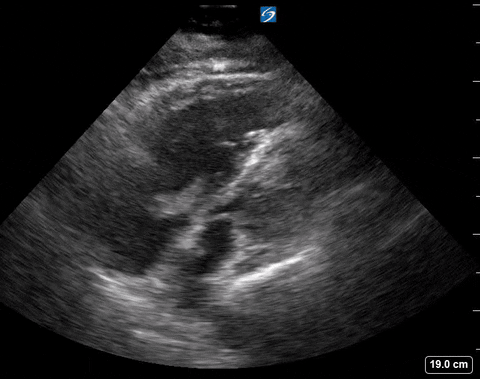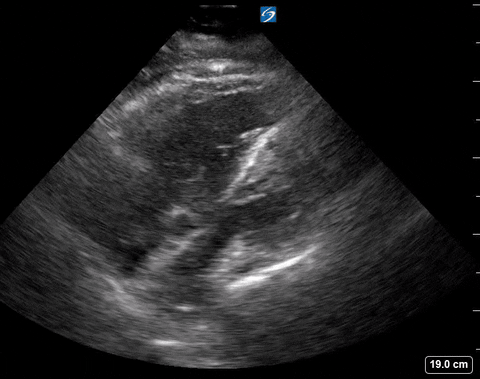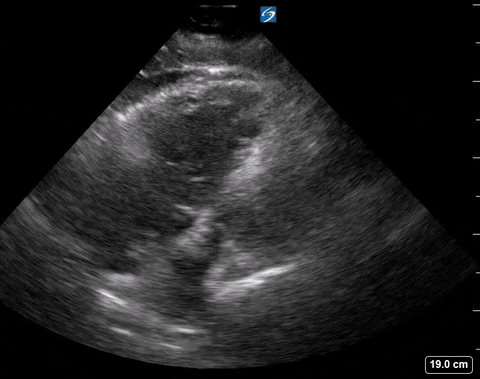
Right heart thrombus
A right heart thrombus appears as a long thin, free-floating wormlike mass. It is differentiated from vegetation or tumour in that it is not attached to the valve.
The authors found based on systematic review and metanalysis that the specificity was 99-100% with very low sensitivity (4.7-5%) and prevalence (1.8-8.7%). The prevalence increased slightly in hemodynamically unstable patients and patients in the ICU.
Right ventricular free wall thickness
With acute RV strain the RV has not had time to adapt to the high right-sided pressure by hypertrophying so when the RV dilates the wall is thin.

The authors found that RVFWT on the SXLA ranged from 3.3-4mm in normal patients and 5.6-11mm in patients with chronic RV dysfunction. The sensitivity, and specificity based on patient databases found that RVFWT is 90-93% sensitive and 94-95% specific for diagnosing chronic pulmonary hypertension7.
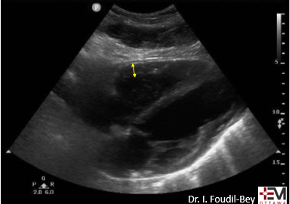
McConnell’s sign
In the original paper, McConnell’s sign was defined as a “distinct regional pattern of RV dysfunction, with akinesia of the mid-free wall but normal motion at the apex”8. The proposed mechanism is that in acute PE, the RB balloons out but the apex is tethered to the hyperdynamic LV giving an appearance of hypokinesis of the free wall and hyperdynamic apex9.
McConnell’s Sign
In chronic PHTN the RV has hypertrophied over time and is not pulled inward as easily. The authors found based on systematic review and meta-analysis that McConnell’s sign was 97-99% specific but only 22-29% sensitive for diagnosis PE. Both sensitivity and specificity increased the more proximal the PE was.
Right atrial enlargement
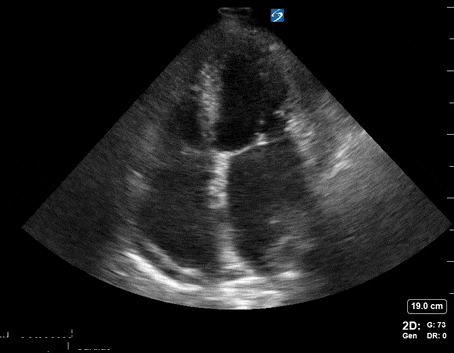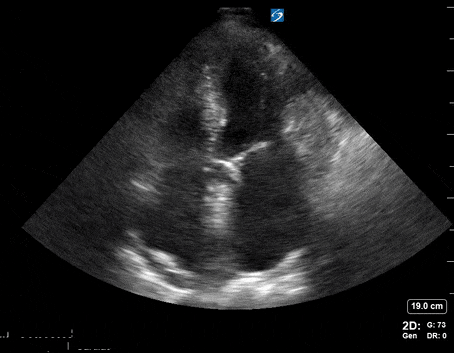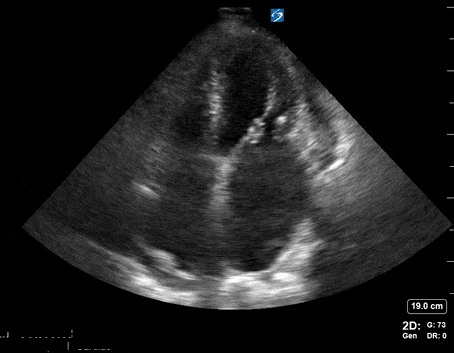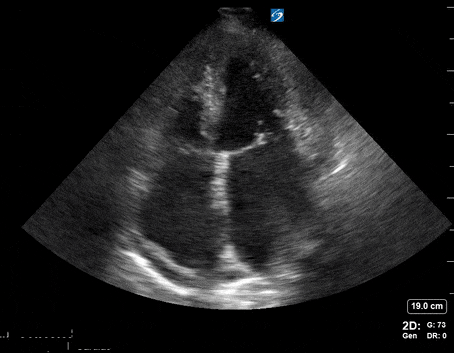
Right atrial enlargement
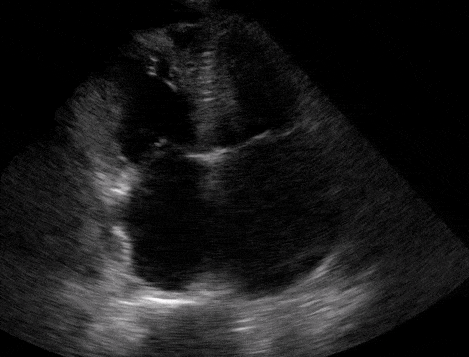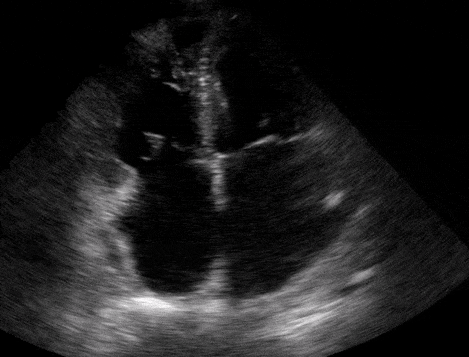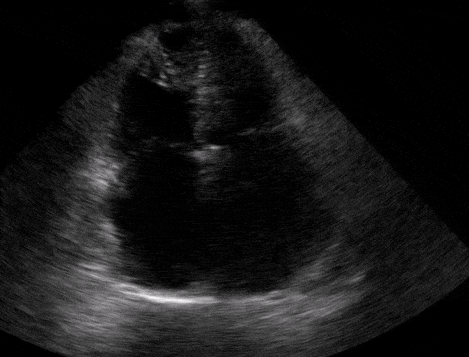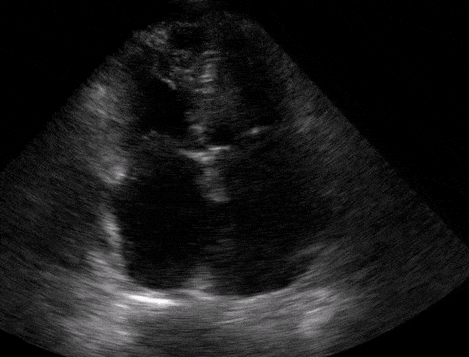
Right Atrial Enlargement
In normal physiology, the RA is normally smaller than the LA so the RA: LA ratio is < 1. In acute RV dysfunction, the RA will dilate so the RA: LA ratio becomes equal to 1. In chronic pulmonary hypertension, the RA typically dilates causing the RA: LA ratio to be >1.

Dr. Murray’s Recommendation
- None of these tests can rule out PE. They only have the specificity to rule it in
- Visualization of a right-sided thrombus is the only test that can be singularly used to rule PE in
- RHT and McConnell’s sign have the highest specificity and can be used as your “go-to’s” to rule in PE.
- Right Ventricular Free Wall Thickness has good sensitivity and specificity for chronic pHTN but neither rules a PE in or out. It can only be used in conjunction with other findings to suggest that it is less likely that there is a PE.
Use of PoCUS to Diagnose Necrotizing Fasciitis
A 24-year-old female who is immunocompetent presents with right thigh swelling, pain and fever. You do a quick PoCUS, and you wonder if your findings can help in the diagnosis of the more sinister soft tissue infection of necrotizing fasciitis.
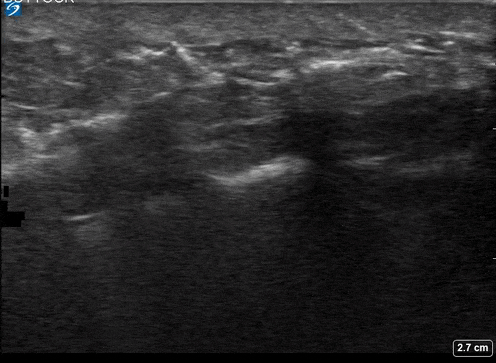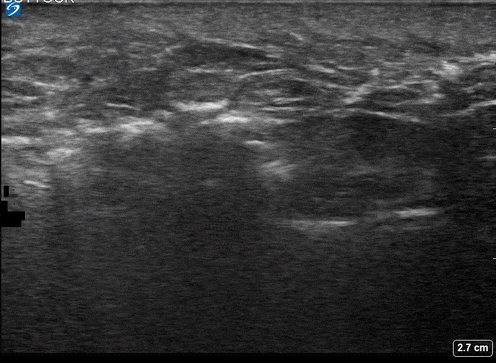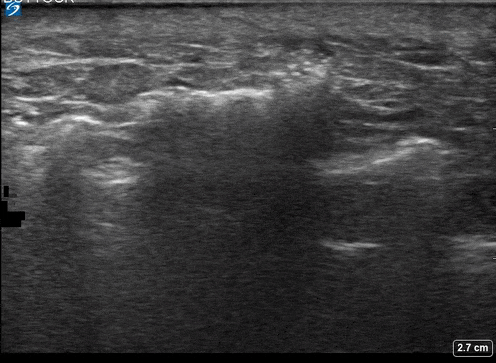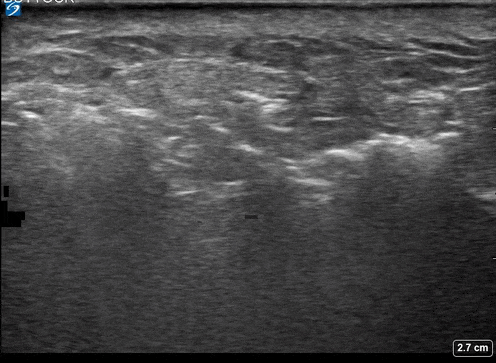
Area of interest #1
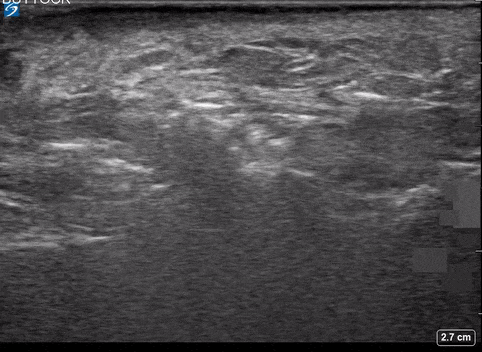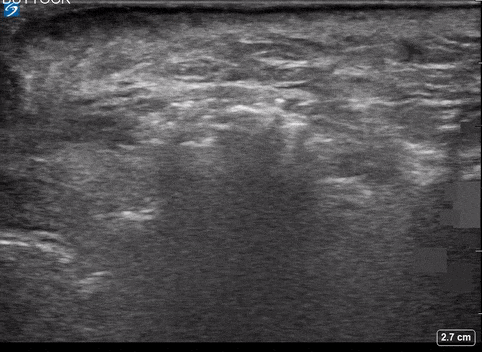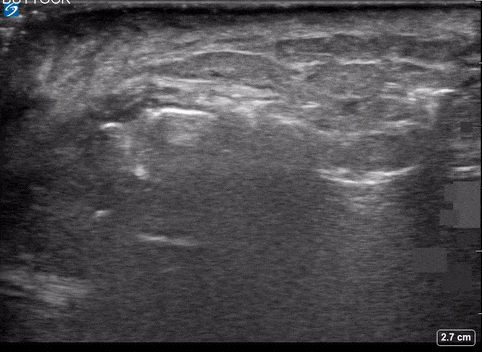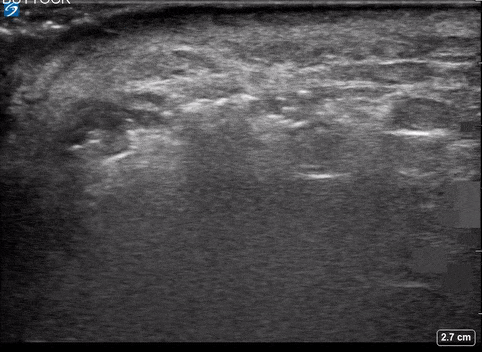
Area of Interest #2
Article 1
This was the first of 2 studies published in 2023 on the use of ultrasound to diagnose necrotizing fasciitis. While there was overlap in the 2 papers regarding 3 larger studies, this specific paper also included an analysis of the case reports in the literature.
Methods
- Extensive literature search with exclusion and inclusion criteria
- 2 independent reviewers with a third to settle discrepancies.
Results
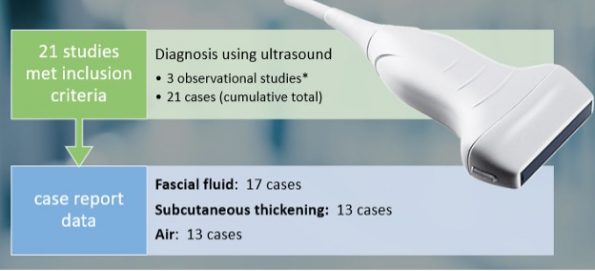
Using the linear probe over infected tissue the most common finding was fascial fluid. Tissue thickening and air were tied for the second most common findings10.
Strengths
- Included the highest quality evidence in addition to case reports.
Limitations
- English papers only
- Publication bias
- A large portion of findings are based on case reports.
Article 2
Methods
In this systematic review, the authors performed an extensive search strategy using multiple research databases and adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses – Diagnostic Test Accuracy (PRISMA-DTA) guidelines for systematic reviews using best practice guidelines. There were 2 independent reviewers with a third resolving discrepancies and articles were graded on the risk of biases.
Results
3 studies were included in the qualitative analysis (one from America and two from Taiwan). In total, there were 221 patients with 73 of them having necrotizing fasciitis. After creating 2×2 tables to calculate test characteristics they found that in general ultrasound had a sensitivity of 85.4%-100% and an of specificity 44.7%-98.2%11.
In terms of specific characteristics, only one study looked at specific findings.
Lin et al. in 2019 reported accuracy stratified by individual ultrasound findings12.
- Fluid accumulation along the fascia
- most sensitive
- 4%(95% CI 72.2% – 93.9%)
- a previous study also demonstrated that the presence of subcutaneous thickening >4mm sascial fluid was 2% sensitive and 93.3 specific for necrotizing fasciitis.
- most sensitive
- Subcutaneous emphysema
- Most specific
- (100%; 95% CI 92.5% – 100%)
- Note: from the previous study fascial fluid and subcutaneous emphysema were the two most common findings in case report data.
- Most specific
Strengths
- Extensive search strategy
- Screened for risk of bias in each article.
Limitations
- All 3 studies used convenience sampling.
- Different diagnostic criteria used to define necrotizing fasciitis on ultrasound.
- All 3 studies had unclear levels of blinding.
Dr. Murray’s Recommendation
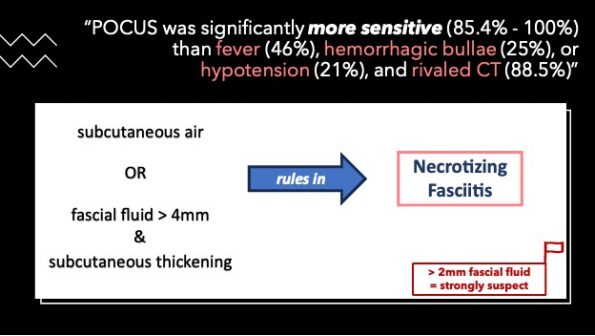
To date, this is the best evidence that we have regarding the use of PoCUS in the diagnosis of necrotizing fasciitis. PoCUS was significantly more sensitive (85.4% to 100%) than fever (46%), hemorrhagic bullae (25%), or hypotension (21%), and the sensitivity rivalled that of CT (88.5%)
If you have a patient with “severe” soft tissue infections take your linear probe.
If you find subcutaneous air this is often a late finding and is the most specific finding for ruling in necrotizing fasciitis.
As you scan you are more likely to see fascial fluid. If this is more than 2 mm you should strongly consider the diagnosis of necrotizing fasciitis, but if it is more than 4 mm with subcutaneous thickening, this finding is highly specific for ruling in the diagnosis.
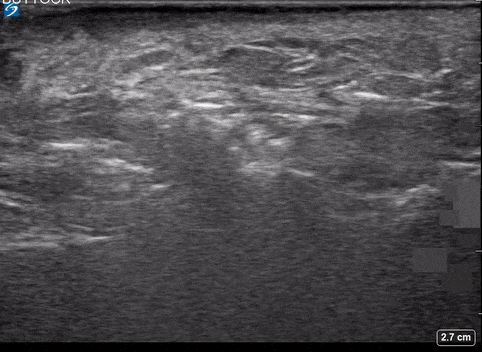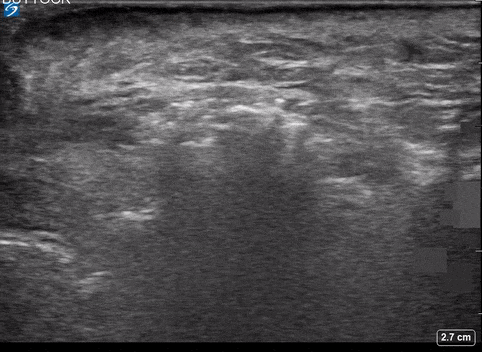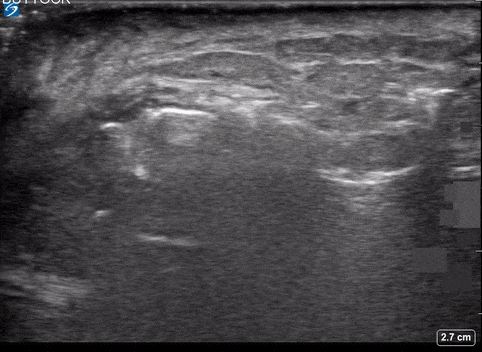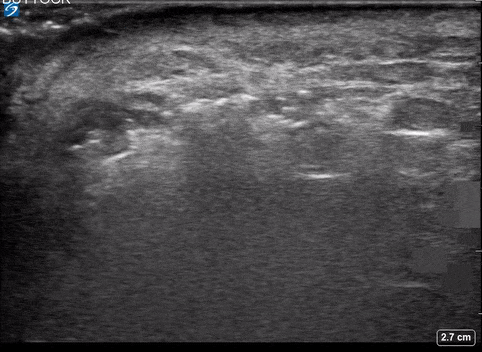
Subcutaneous emphysema
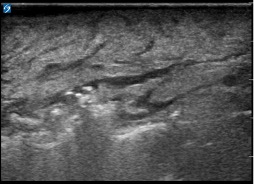
Fascial fluid
PoCUS for Chest Tube Landmarking
A 22-year-old female presents to the ER with sudden onset shortness of breath & pleuritic chest pain. You perform a POCUS and see the absence of lung sliding. An x-ray is also performed.
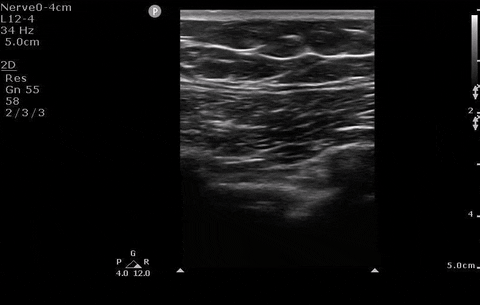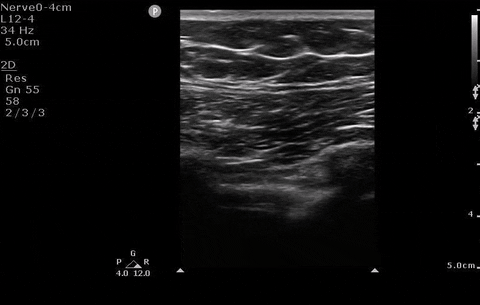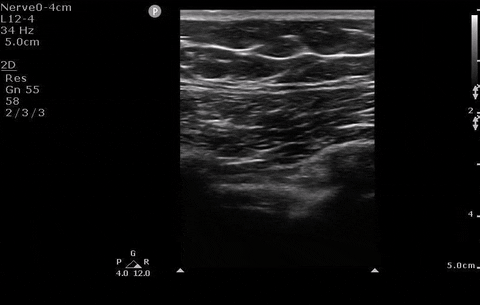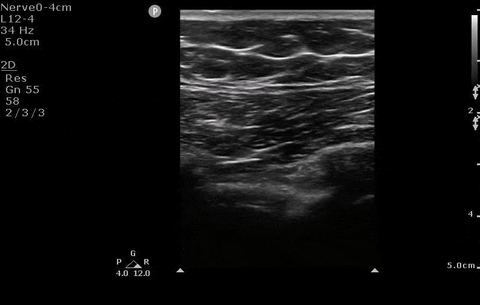
Pleural assessment of right lung
You correctly identify that this patient has a pneumothorax (and because you’ve read our post on pneumothorax management ) you recognize that this patient needs surgical management. You bring your chest tube equipment to the bedside and landmark in the triangle of safety at the level of the inframammary fold as you have been trained to do in preparation for a chest tube.
You feel it looks a “bit low”. Are there any tools you can you to improve the safety of your procedure?
Methods
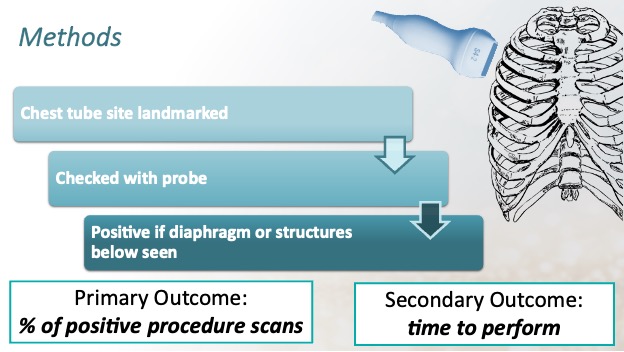
Observational study of adult emergency department patients recruited through a convenience sample.
Treating EM residents and attending physicians were asked to rapidly assess and mark the chest wall with a surgical pen using a landmark technique where they would theoretically insert a chest tube.
A PoCUS fellow then placed the phase array probe in the center of the site and observed through one single respiratory cycle.
- Primary outcome
- Percentage of PoCUS thoracic procedures scans that identified soft tissue structures such as the diaphragm plus liver or spleen over the regular respiratory cycle in the intercostal space selected for the tube thoracostomy.
- Secondary outcomes
- Average time required to perform PoCUS scan14.
Results
A total of 76 quick looks with PoCUS
- 17% demonstrated soft tissue (diaphragm, liver or seen) (13/76) at the marked site.
- Average time for a quick look through the entire respiratory cycle = 43 seconds.
- Scans were then reviewed by a blinded ultrasound director with high inter-rater reliability (95%).
Strengths
- high inter-rater reliability in terms of interpretation of the scans.
Limitations
- Small study.
- The majority of participants were residents (but had done ATLS).
- 18.9% of participants who marked below the diaphragm were senior EM residents.
Dr. Murray’s Recommendation

Concluding Thoughts
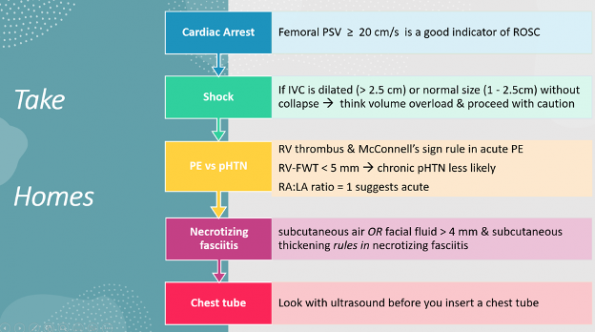
The use of PoCUS in a variety of EM presentations is gaining a lot of traction. This is best demonstrated by the expanding body of literature that is becoming available. In this article, Dr. Murray highlighted 5 articles published in 2023 that he thought had the most potential to benefit your practice. His recommendations were detailed above, but here are the take-home points.
References


